Optimizing Therapeutic Sperm Washing Medium: Why are there clear differences in sperm progression and velocity between products?
There are numerous commercially available sperm washing and resuspension buffers (SWBs) used for sperm preparation and insemination. All are widely considered to provide similarly suitable conditions to support sperm function prior to assisted reproduction. This article provides an overview of our work exploring SWB formulations to identify whether we can optimize SWB composition in relation to two factors that we have identified as potentially important – namely, pH and bicarbonate.
Essential properties of SWBs include balancing the requirement for long-term motility and capacitation support with the need to ensure that the acrosome reaction does not happen prematurely, and that sperm do not metabolically ‘burn-out’ too early. As there is a general assumption that the majority of SWBs perform to a similar standard, product choice from the perspective of the embryology laboratory is often simply influenced by cost, or by an historical or personal preference. Yet this idea that performance does not vary between commercial SWBs may be because subtle but important differences between products exist that are difficult to detect using manual motility estimates.
Furthermore, sperm velocity, measured linearly or curvilinearly, has been consistently demonstrated to be fundamental to predicting conception1,2, either natural or assisted. As such it should always be considered when evaluating SWB performance. Arguably, manual estimates of sperm velocity are subjective and lack standardization; for reliable assessments a suitable computer assisted sperm analysis, or CASA, system provides an accurate technical alternative3. To explore the parameters of SWBs that affect sperm motility and velocity, for this study we chose to use the SAMi software system.
Important Parameters For Sperm Function
As part of a performance review/validation exercise in our laboratory, we determined that washed-prepared donor sperm demonstrated subtle differences for both percent progression and progressive velocity when incubated with three commercially available media. This finding prompted further investigation since it potentially has implications for the unit’s relative success of IUI using partner or donor sperm. In terms of basic solute composition, osmolality, and viscosity, there appeared to be few differences between products. However, two factors stood out as potential targets for further investigation: bicarbonate content and pH.
Both of these parameters have, for some time, been known to play an important role in suppressing sperm motility while they mature in storage in the epididymis, and in their activation and hyperactivation3,4,5. However, high bicarbonate levels have been associated with metabolic burn-out. Furthermore, manufacturers’ data indicate that wide variations exist between SWBs in terms of pH stability. Understanding the effects of bicarbonate concentrations and pH on sperm in vitro with respect to sperm motility and velocity is therefore important if we are to optimize our use of sperm washing buffers.
How Did We Investigate?
To investigate the effect of pH and bicarbonate on sperm progression and motility, six pharmaceutical-grade test media were formulated (by CooperSurgical Research and Development) using the same base medium, but with differing pH (7.0-8.5) and different bicarbonate levels (0-30mmol). Human serum albumin (HSA) concentration was kept constant across all formulations. These media were tested before use and at the end of the test period of six weeks to determine pH change.
Testing involved preparation of sperm from 20 donors with proven fertility using our standard density gradient; each donor’s sperm samples were then resuspended in each of the six test media formulations. Each experiment was therefore internally controlled by exposing the same test-sample to each media combination. Sperm motility and curvilinear velocity were then quantified using the SAMi CASA system (Procreative Diagnostics) at 0, 3, 6 and 24 hours. A single technician blind to media composition during testing performed all 20 experiments.
What We Have Found So Far
Data from our de facto pH tests indicate that pH levels did vary with time for the test media (see Figure 1), with pH increasing across all formulations, but that all test media formulations were relatively stable over the six-week testing period in this respect.
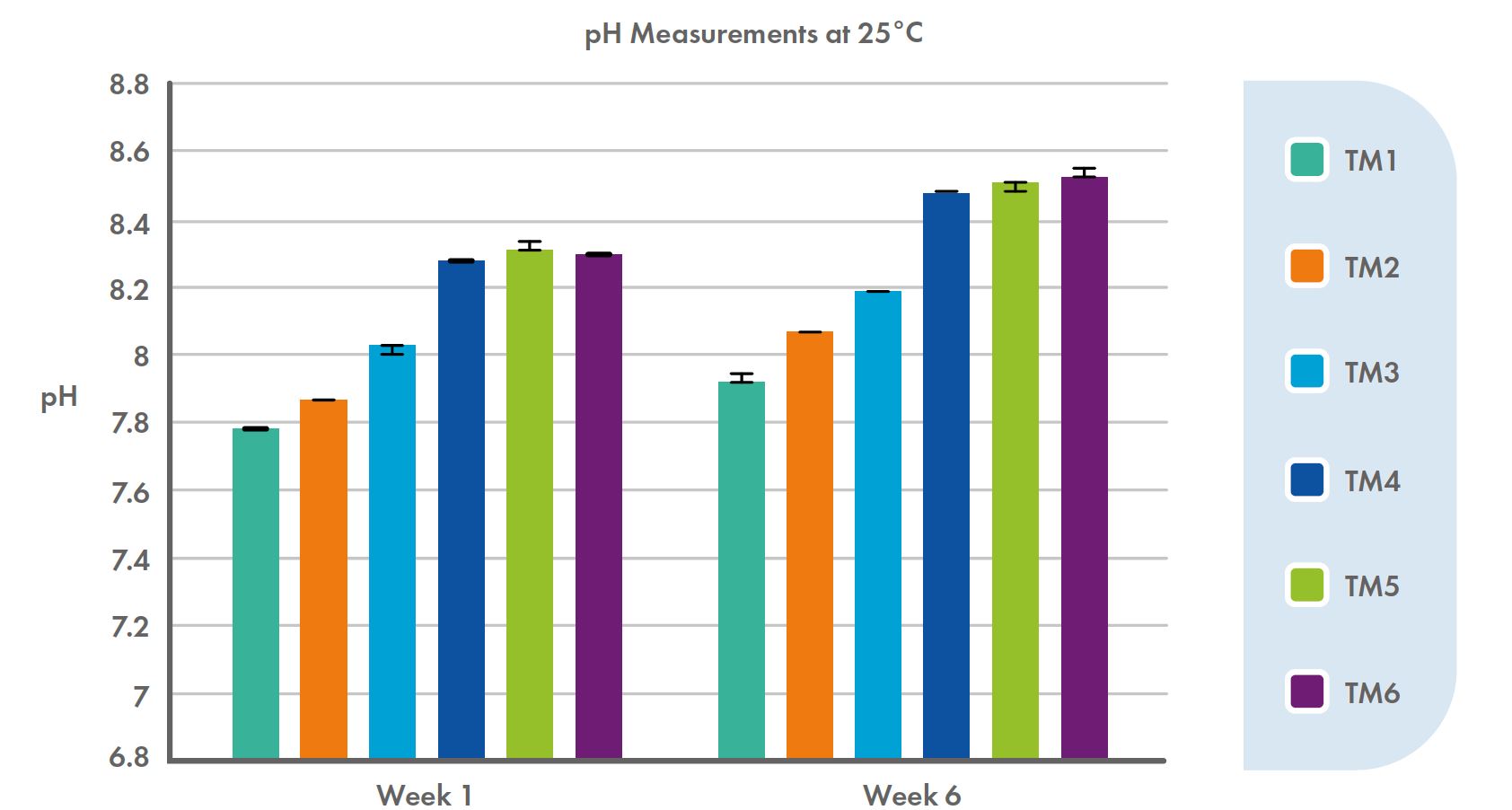
Figure 1: pH of the six test media (TM1 – TM6) tested at week one and week six post-production of six TM media.
Regarding progressive motility, significant differences were observed between the test media. Analysis also revealed significant correlations for both progression and velocity with pH and bicarbonate levels over time: High pH, high bicarbonate (or both) maintained sperm with the highest percent sperm progression and velocity (Figure 2).
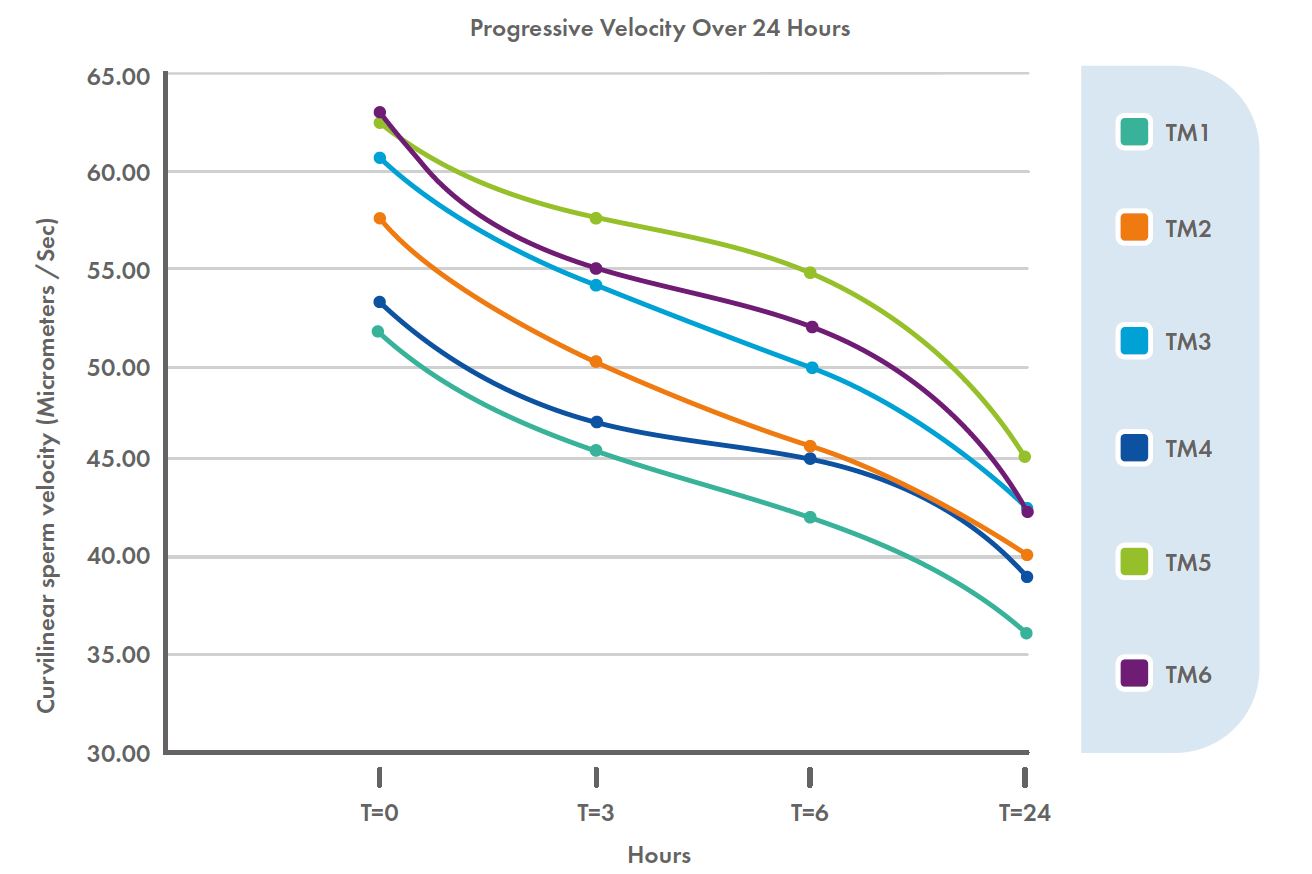
Figure 2: Progressive curvilinear sperm velocity (µm/s) for six test media over 24 hours.
Our Initial Conclusions
The ‘take-home’ message is both simple yet significant, and one which is certainly not universally appreciated. This straightforward but effective experiment demonstrated that either pH, bicarbonate (or both acting synergistically) is important for sustaining a high level of sperm motility and velocity in vitro.
Six test derivatives from the same base medium but with differing pH and containing different levels of bicarbonate were tested for influence on sperm motility and curvilinear velocity. Both these variables were shown to correlate with the formulation changes made. These results may have implications for SWB composition for both clinical and laboratory applications, and for diagnostic semen analysis.
A number of authors have utilized CASA to demonstrate the importance of sperm swimming speed for establishing fertilization and pregnancy, and have suggested that simple reporting of progressive motility provides an ‘incomplete picture’ when it comes to assessing a sperm sample’s viability in terms of potential IVF success.2,6,7,8. Therefore, it would seem logical that we should take into account both sperm motility and velocity as viable measures when attempting to improve SWB composition by varying factors (such as pH and bicarbonate levels) that are known to affect these functions in ways that impact on oocyte fertilization. Furthermore, as CASA systems appear to provide a reliable means of quantifying sperm motility and velocity, their consistent use in research into media optimization would allow for better data comparison across studies.
Although many laboratories would not contemplate incubating sperm for extended periods, there may be situations where it may be useful; overnight incubation where insemination has not been possible, for example. Also, perhaps, extended incubation could be useful for comprehensive toxicity testing of laboratory consumables9 or even healthcare products, such as specimen collection condoms or gel lubricants10.
One concern was that excessive bicarbonate could have a detrimental effect on sperm function longer term, leading to ‘burn out’ or over-performance with a subsequent loss of essential nutrients required to maintain a high level of motility. This issue was not observed experimentally, nor was there any evidence of adverse effects on other end-point measures such as the acrosome reaction.
Follow-up experiments are planned which should help to optimize the pH and bicarbonate composition of SWBs that both maximizes motility/velocity yet minimizes the potential of sperm ‘burn out’ due to increased metabolism. Overall, our preliminary work indicates that more needs to be understood about optimal SWB formulation.
New research on Andrology has been published!
PICSI® Dish and SpermSlow™ Media
by Steve Fleming PhD – Director of Embryology, CooperSurgical

References
- Tomlinson M, Lewis S, Morroll D; British Fertility Society (2013). Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil (Camb). 16(3):175-93.
- Tomlinson M and Naeem A (2018). CASA in the medical laboratory: CASA in diagnostic andrology and assisted conception.
Reprod Fertil Dev. 30(6):850-859 - Okamura N, Tajima Y, Ishikawa H, Yoshii S, Koiso K, Sugita Y (1986). Lowered levels of bicarbonate in seminal plasma cause the poor sperm motility in human infertile patients. Fertil Steril. 45(2):265-72.
- Shum WW, Ruan YC, Da Silva N, Breton S (2011). Establishment of cell- cell cross talk in the epididymis: control of luminal acidification.
J Androl. 32(6):576-86. - Ickowicz D, Finkelstein M, Breitbart H (2012). Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl. 2012 14(6):816-21.
- Barratt CLR, Tomlinson MJ and Cooke ID (1993). Prognostic significance of computerized motility analysis for in vivo fertility. Fertil. Steril. 60, 520-525.
- Garrett, C, Liu DY, Clarke GN, Rushford DD and Baker HW (2003). Automated semen analysis: ‘zona pellucida preferred’ sperm morphometry and straight-line velocity are related to pregnancy rate in subfertile couples. Hum. Reprod. 18,1643-1649.
- Shibahara, H, Obara H, Ayustawati, Hirano, Y, Suzuki T, Ohno A, Takamizawa S and Suzuki M (2004). Prediction of pregnancy by intrauterine insemination using CASA estimates and strict criteria in patients with male factor infertility. Int J Androl. 27(2):63-8.
- Long S, Woodward B and Tomlinson M (2017). Sperm toxicity testing: UK best practice guideline from the Association of Biomedical Andrologists. Br. J. Biomed Sci. 75(2):53-60.
- Tomlinson M, Naeem A, Hopkisson JF, Campbell B (2012). Brief communication: assessment and validation of nonspermicidal condoms as specimen collection sheaths for semen analysis and assisted conception. Hum Fertil (Camb). 15, 140-143.

 Mathew Tomlinson PhD
Mathew Tomlinson PhD
Consultant Scientist in Andrology;
PR for NUH Life Fertility Services, and Lecturer in ART (University of Nottingham).
Mathew Tomlinson PhD is Clinical Scientist in Andrology at Nottingham University Hospitals. After completing a PhD on male infertility in Sheffield in 1992, he worked first at the Royal Shrewsbury Hospital before moving onto Birmingham Women’s Hospital to develop region-wide andrology services in a more academic setting. A move in 2004 to Nottingham University Hospitals saw him develop his interests in education, cryopreservation and, more latterly, automation in semen analysis.
Dr Tomlinson is now HFEA Person Responsible for the regional NHS Fertility unit, plays an integral role on the Masters course in Assisted Reproduction and runs training workshops in andrology. He continues to focus on a holistic and evidence-based approach to diagnosing and treating infertility and has varied research interests. He has published over 50 papers and chapters in Clinical Andrology and Assisted Reproduction.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada