Introduction
One of the greatest unmet challenges in Assisted Reproductive Technology is to formulate an IVF embryo culture medium that accurately reflects the natural conception environment. Among the elements present in vivo but missing from most commercial culture media are polypeptide cytokines, or cytokines.
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is a cytokine that has been demonstrated in extensive preclinical and clinical research to promote development of healthy human embryos. After natural conception in vivo, GM-CSF is secreted into the fallopian tube and uterus and is present in the fluid bathing the developing embryo.
Receptors for GM-CSF are expressed on the surface of the embryo from the zygote through to implanting blastocyst stage and, after implantation, trophoblast cells also express GM-CSF receptors. In humans, clinical trials have shown that GM-CSF can promote blastocyst development and increase the chance of implantation success, particularly for some subsets of women.
In a natural conception in vivo the embryo is exposed to a changing mixture of cytokines as it moves through the oviduct and uterus before implantation. Cytokines act as signalling agents that mediate communication between the embryo and maternal tissues, regulating synchronous development and maximising the chances of implantation success.
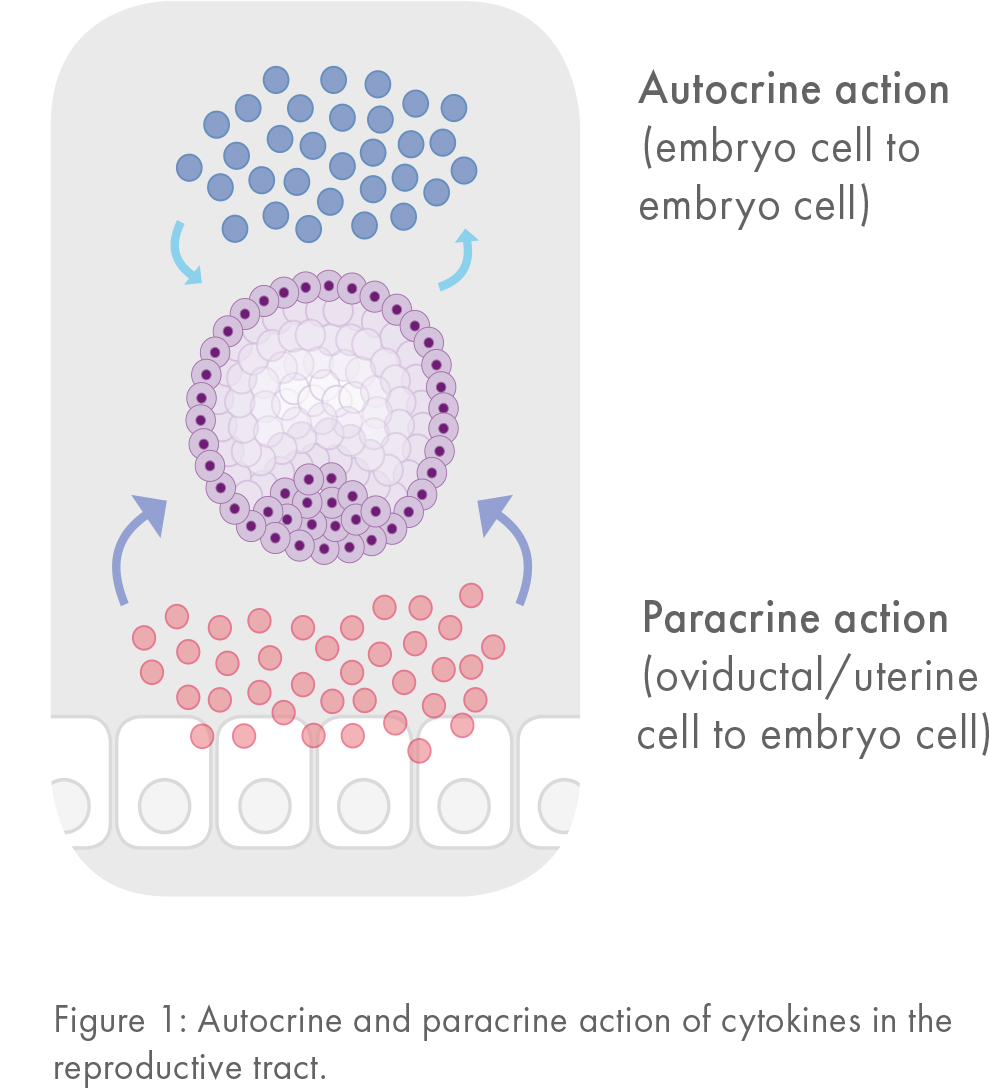 Although embryos can develop in vitro in simple culture media, there is good evidence that better developmental potential is achieved when embryos are exposed to cytokines in the natural conception environment.1,2 These cytokines fine-tune gene expression and protect embryos from cell stress, which in turn promotes cell survival and on-time development. The result is to increase implantation competence and development of a robust placenta.1,3,4
Although embryos can develop in vitro in simple culture media, there is good evidence that better developmental potential is achieved when embryos are exposed to cytokines in the natural conception environment.1,2 These cytokines fine-tune gene expression and protect embryos from cell stress, which in turn promotes cell survival and on-time development. The result is to increase implantation competence and development of a robust placenta.1,3,4
A wide range of cytokines promote preimplantation embryo development. Many of these cytokines stimulate overlapping signaling pathways. These so called ‘autocrine’ factors explain why IVF embryos grow better when cultured in groups and in small volumes of culture media (Figure 1).
Cytokines do not stimulate the mitotic cell cycle; rather, they act as survival agents to prevent cells from constitutive death pathways mediated by p53-induced apoptosis.5
However, cytokines do not completely prevent apoptosis in developing embryos.6 A certain level of apoptosis in blastocysts is considered normal in both in vivo and in vitro developed embryos, since apoptosis is a necessary biological mechanism for elimination of abnormal or unwanted cells in development.7 There is no evidence that cytokines rescue abnormal cells, and such an action would seem unlikely given that no such function of cytokines is seen in other cell types.
Even with best practice and conditions to promote autocrine cytokine actions, embryos develop at a slower rate and with fewer cells in vitro than in vivo.2 This demonstrates the crucial role of paracrine factors normally secreted by maternal tissues (Figure 1). In vivo, cytokines released from oviductal and uterine cells bathe the developing embryo as it traverses the reproductive tract prior to implantation. Maternal factors act in concert with autocrine mediators to modulate the endogenous developmental program.1
GM-CSF, a key cytokine regulator
There is one cytokine that embryos cannot make on their own, and this is Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF, also known as CSF2). In women, GM-CSF is secreted by fallopian tube and uterine epithelial cells in maximal amounts during the mid-luteal phase.8,9
GM-CSF operates as a survival factor in embryos, promoting normal glucose uptake10 and providing protection from the default pathway of stress response and apoptosis that occurs in the absence of cytokines11 (Table 1 and Figure 2).
| Physiological Effect of GM-CSF | Change |
|---|---|
| Zygote progression to blastocyst | Increased |
| Time to develop to blastocyst | Faster |
| Cell number and allocation to ICM and TE | Normalized |
| Cell viability and apoptosis | Normalized |
| Gene expression profile | Normalized |
| Glucose uptake | Increased |
| Stress response | Decreased |
| Implantation and developmental competence | Increased |
Table 1
The GM-CSF response may interact with environmental stressors, since GM-CSF appears most effective in sustaining blastocysts when stress is induced by omitting Human Serum Albumin (HSA) from the culture medium.12 One role of albumin is to act as a carrier for autocrine cytokines that sustain embryo development.
In human IVF embryos, in vitro culture can lead to arrested, delayed and abnormal cell division with often fewer than 50% of embryos reaching the blastocyst stage. Embryotrophic effects of GM-CSF are seen in human embryos13, with addition of GM-CSF substantially increasing the proportion of frozen thawed embryos developing to blastocysts.6,13 The developmental competence of blastocysts, assessed by hatching and attachment to extracellular matrix-coated culture dishes, also shows improvement after GM-CSF exposure.13 Even in the presence of other cytokines, GM-CSF has an important role; when human IVF embryos were co-cultured with autologous endometrial cells, production of GM-CSF correlated with likelihood of successful pregnancy after embryo transfer.14
The cytokine environment provided by the female reproductive tract at conception can be a major factor in programming later fetal and placental development. Even small perturbations in blastomere number and inner cell mass/trophectoderm allocation in the blastocyst influence the growth trajectory of the fetus and offspring.15
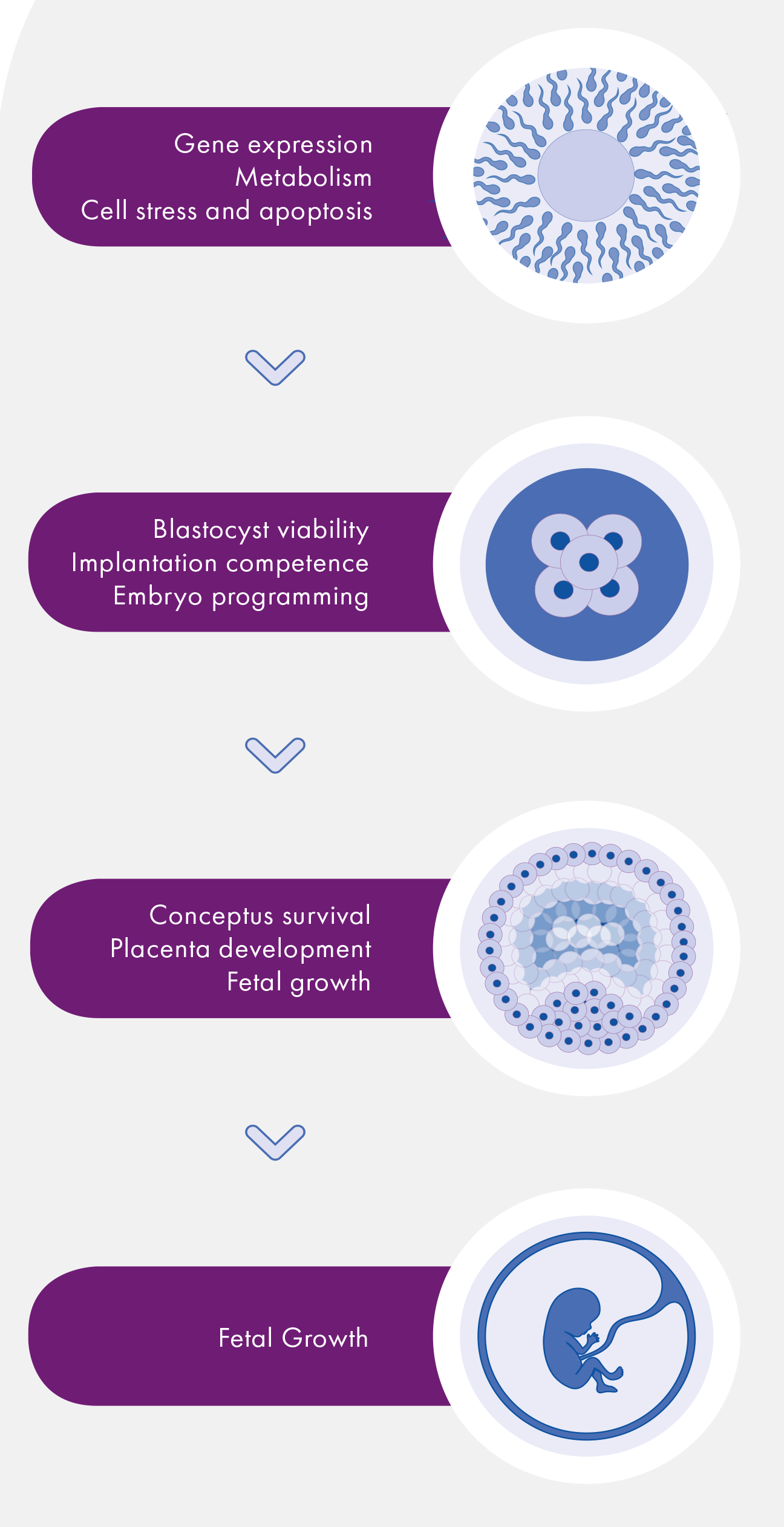
Figure 2: Cytokine expression in the female reproductive tract is regulated by ovarian steroid hormones and a variety of intrinsic and extrinsic factors. The cytokine environment in the periconception period is a balance between embryotrophic (anti-apoptotic) and embryotoxic (apoptotic) factors. Together these factors influence gene expression, metabolism, cell stress and apoptosis pathways in the preimplantation embryo to initiate downstream events impacting implantation, placental development and fetal growth, and ultimately the phenotype and health of progeny.
Clinical studies of GM-CSF in IVF embryo development
A study using human IVF embryos prepared from donor oocytes confirmed that no increase in chromosomal abnormality was evident in embryos cultured with GM-CSF.16 Culture with GM-CSF resulted in 34.8% uniformly normal embryos, compared with 33.3% uniformly normal embryos in the absence of cytokine. Thus, it appears highly unlikely that GM-CSF ‘rescues’ chromosomally abnormal blastomeres or embryos. To evaluate the effect of adding GM-CSF to human IVF embryos on implantation rate, a multicenter, randomized, placebo-controlled, double-blinded prospective study was completed in 2007. It involved 1,332 Danish and Swedish patients recruited from 14 fertility clinics. Fertilization, embryo culture until Day 3 and embryo transfer utilized either control medium, or medium supplemented with 2 ng/ml GM-CSF. GM-CSF gave a significant increase in implantation rate at gestational week 12, with an ongoing implantation rate (number of fetal heartbeats expressed as a ratio of embryos transferred) of 23.0% for the GM-CSF group, compared with 18.7% for the control medium.17 This led to a higher likelihood of live birth, with no adverse effects of GM-CSF on stillbirth, perinatal death, or fetal abnormality, while gestational length and birth weight were unchanged.17
In the first 620 women treated, a base medium containing 2 mg/ml HSA was used. This was changed to a base medium containing 5 mg/ml HSA for the next 529 women following an interim review. The effects of GM-CSF interacting with the base medium, showed a significant effect only with low HSA. Since low HSA protein is a significant stressor, this is consistent with GM-CSF protecting embryos from culture-induced stress.
The most profound effect of GM-CSF was evident in a subgroup of 327 women with previous miscarriage after IVF or natural conception, where ongoing implantation rates at week 12 increased by 40%, from 16.5% in the control medium to 23.2% with GM-CSF. A follow-up of children born in the completed study revealed that GM-CSF increased the number of women achieving a live birth in the miscarriage subgroup by 28%, from 23.1% to 29.6%, and this effect was independent of the HSA content of culture medium (Figure 3).
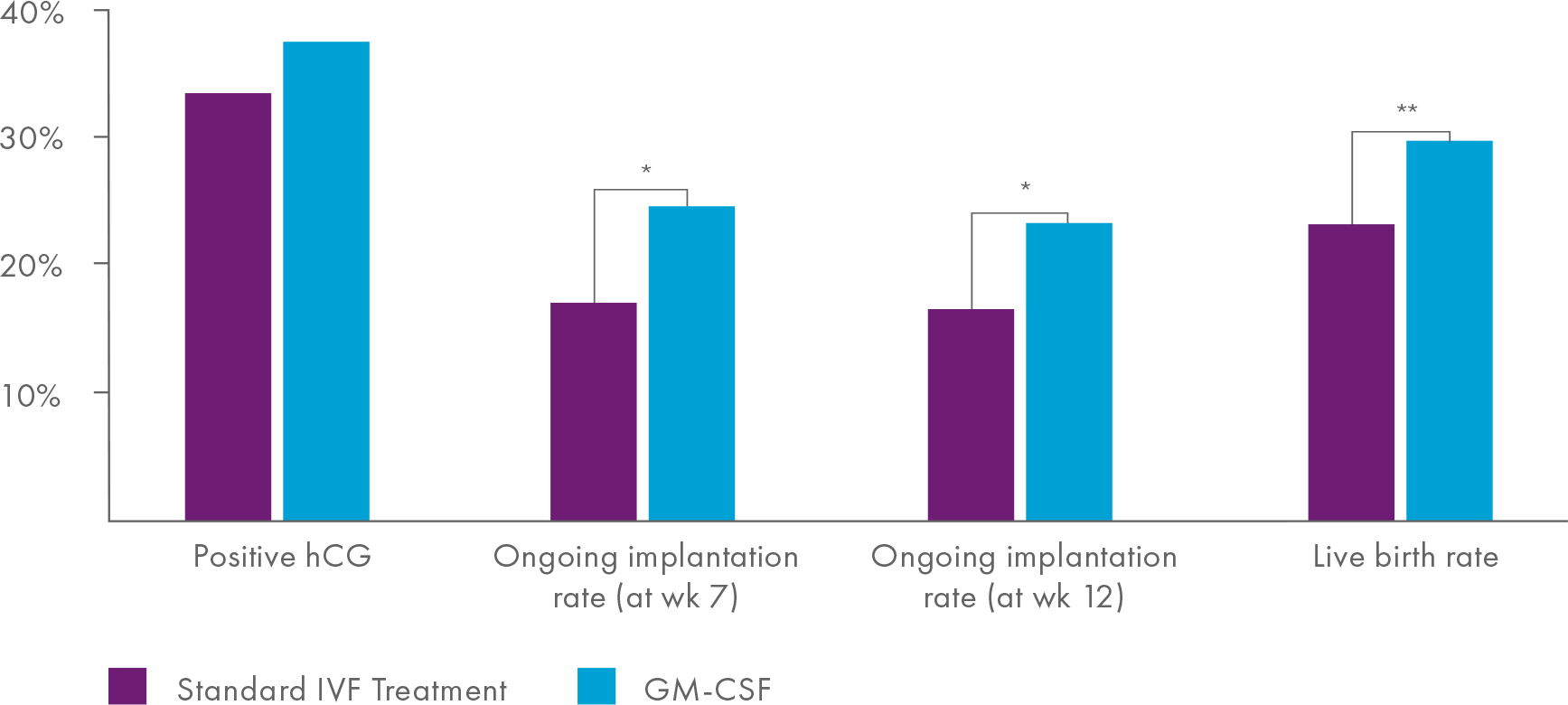
Figure 3
It was notable that the overall biochemical pregnancy rate was not altered with GM-CSF. Rather, GM-CSF treatment was linked with sustained ongoing implantation and reduced fetal loss prior to week 12, with fetal loss before 12 weeks decreasing from 33.5% to 22.9% of all women with positive human Chorionic Gonadotropin (hCG).
This large clinical trial provides evidence that, under some circumstances, there are benefits of GM-CSF in IVF embryo culture media for successful pregnancy and a healthy infant at term. In some women, the maternal tract may be less capable of supporting embryo development, or embryos are more vulnerable to culture-induced stress. Importantly, there was no evidence of any adverse outcome in the GM-CSF group, which is reassuring.
Summary
GM-CSF is the first cytokine to have demonstrated utility in human IVF. There is future potential for cytokines in ART, but well-designed research and clinical trials are required to understand the natural biological mechanisms of their actions, and to provide a solid evidence basis for their clinical use.
Conflict of Interest Statement: Sarah Robertson PhD is an inventor on patents concerning utility of GM-CSF in IVF culture medium and receives royalty income from CooperSurgical.
References
1. Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol 2002; 172:221-236.
2. Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro Curr Opin Obstet Gynecol 2008; 20:292-304.
3. O’Neill C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update 2008; 14:275-288.
4. Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA 2014; 111:2200-2205.
5. O’Neill C, Li Y, Jin XL. Survival signaling in the preimplantation embryo. Theriogenology 2012; 77:773-784.
6. Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod 2002; 67:1817-1823.
7. Hardy K. Apoptosis in the human embryo. Rev Reprod 1999; 4:125-134.
8. Giacomini G, Tabibzadeh SS, Satyaswaroop PG, Bonsi L, Vitale L, Bagnara GP, Strippoli P, Jasonni VM. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Human Reproduction 1995; 10:3259-3263.
9. Zhao Y, Chegini N, Flanders KC. Human fallopian tube expresses transforming growth factor (TGF beta) isoforms, TGF beta type I-III receptor messenger ribonucleic acid and protein, and contains [125I] TGF beta-binding sites. J Clin Endocrinol Metab 1994; 79:1177-1184.
10. Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod 2001; 64:1206-1215.
11. Chin PY, Macpherson AM, Thompson JG, Lane M, Robertson SA. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF). Human Reprod 2009; 24:2997-3009.
12. Karagenc L, Lane M, Gardner DK. Granulocyte-macrophage colony-stimulating factor stimulates mouse blastocyst inner cell mass development only when media lack human serum albumin. Reprod Biomed Online 2005; 10:511-518.
13.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod 1999; 14:3069-3076.
14. Spandorfer SD, Barmat LI, Liu HC, Mele C, Veeck L, Rosenwaks Z. Granulocyte macrophage-colony stimulating factor production by autologous endometrial co-culture is associated with outcome for in vitro fertilization patients with a history of multiple implantation failures. Am J Reprod Immunol 1998; 40:377-381.
15. Thompson JG, Kind KL, Roberts CT, Robertson SA, Robinson JS. Epigenetic risks related to assisted reproductive technologies: short and long-term consequences for the health of children conceived through assisted reproduction technology: more reason for caution? Hum Reprod 2002; 17:2783-2786.
16. Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sorensen PD, Ziebe S. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reprod Biomed Online 2010; 20:477-484.
17. Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, Gabrielsen A, Hnida C, Zobel DP, Munding B, Bendz SH, Robertson SA. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro Fertil Steril 2013; 99:1600-1609.
 Professor Sarah Robertson received her PhD in reproductive immunology from the University of Adelaide in 1993. She was an NHMRC Research Fellow in 1996-2013 and has been Director of the Robinson Research Institute, University of Adelaide since 2013. Sarah’s research focus is immune regulation of conception and embryo implantation, and consequences for reproductive success and offspring health. She is funded by the NHMRC, ARC, CIHR and Gates Foundation, and has more than 180 peer-reviewed scientific journal papers and reviews. Elected Fellow of The Australian Academy of Science, the Australian Academy for Health and Medical Sciences, and Fellow of the Society for Reproductive Biology, she was Editor-in-Chief of The Journal of Reproductive Immunology in 2009-2013. Currently, Sarah serves on the Editorial Boards of Endocrinology and the Journal of Clinical Investigation.
Professor Sarah Robertson received her PhD in reproductive immunology from the University of Adelaide in 1993. She was an NHMRC Research Fellow in 1996-2013 and has been Director of the Robinson Research Institute, University of Adelaide since 2013. Sarah’s research focus is immune regulation of conception and embryo implantation, and consequences for reproductive success and offspring health. She is funded by the NHMRC, ARC, CIHR and Gates Foundation, and has more than 180 peer-reviewed scientific journal papers and reviews. Elected Fellow of The Australian Academy of Science, the Australian Academy for Health and Medical Sciences, and Fellow of the Society for Reproductive Biology, she was Editor-in-Chief of The Journal of Reproductive Immunology in 2009-2013. Currently, Sarah serves on the Editorial Boards of Endocrinology and the Journal of Clinical Investigation.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada