We live in an era of amazing technology, where nearly everything we encounter is a sophisticated man-made wonder.
We have complex culture media, recombinant proteins, custom dishes for embryo culture and even incubators with cameras – yet oil remains essentially unchanged since its first use over 50 years ago, and our understanding of its complex nature is changing.
Oil products require quality control¹. With the introduction of peroxide value tests, end users have better assurance that oil today is of high quality. In addition, new analysis methods such as chemical fingerprinting provide the possibility to screen for the presence of potential embryo toxic substances as well as unsaturated carbon chains2.
“Truth will rise above falsehood as oil above water.”
Miguel de Cervantes
So where does oil come from and do names matter?
All oil available commercially for embryo culture today is what experts in petroleum science call “white oil”. When crude oil is subjected to intense heat, it boils and separates into fractions that are collected based on boiling point. The fraction that we care about – white oil – has a boiling point >200°C and is processed to render it mostly inert. Additional processing and testing is performed to yield a pharmaceutical grade and an industrial grade.
Pharmaceutical grade means the oil is suitable for clinical use in humans, most commonly as an intestinal lubricant. Pharmaceutical white oil goes by two names in the ART world – mineral oil and paraffin oil. According to the monographs from the United States Pharmacopeia (USP) for mineral oil and the European Pharmacopeia (EP) for paraffin oil, the two oils are exactly the same with the same chemical abstract # (CAS 8042-47-5).
One oil, two names. If you’re told otherwise, ask for proof, as it is most likely merely a difference in marketing.
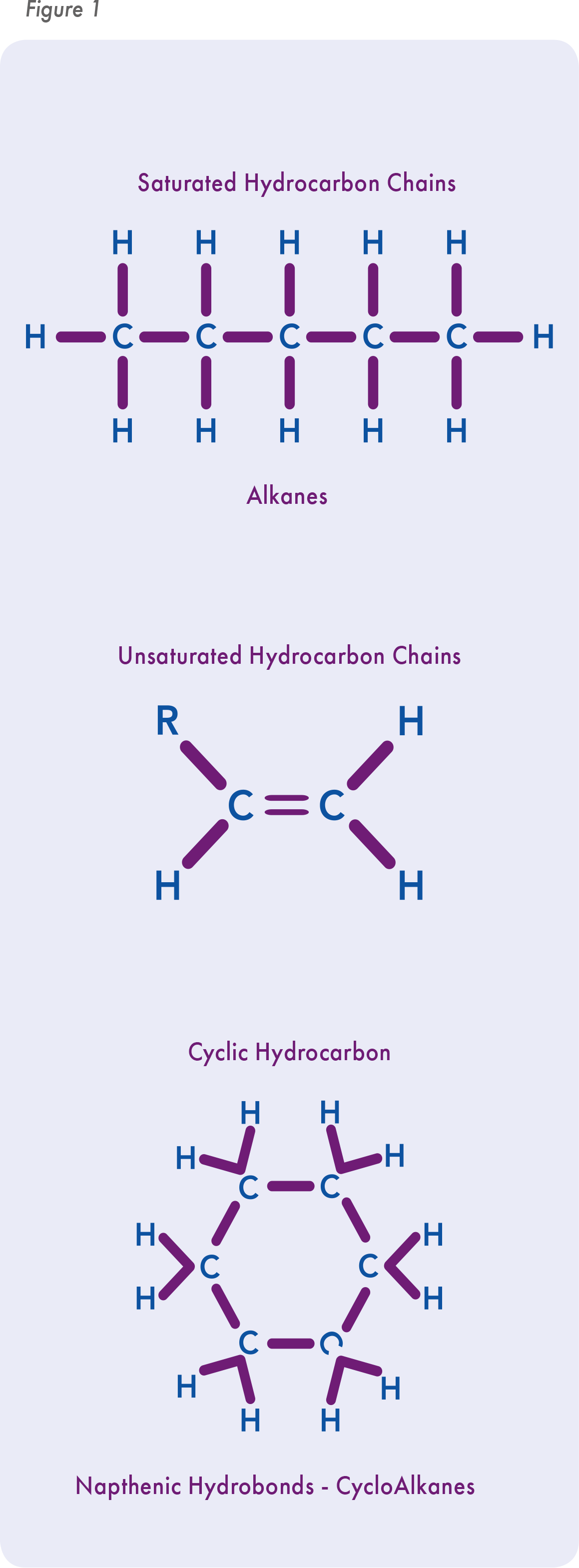
The reality is that both paraffin and mineral oil are mixtures of straight chain hydrocarbons (alkanes; paraffin) and cyclic hydrocarbons (cyclo-alkanes; naphthenic). These two oil components share one very important trait – they are by definition fully saturated, making them very unlikely to react with oxygen and become reactive. Mineral and paraffin oil used in the ART industry contain roughly 70% paraffinic and 30% naphthenic hydrocarbons. The quality of the oil is dependent in part on its inertness, i.e. the degree of saturation.
While there are methods available to fractionate white oil into pure paraffin, the process is difficult and expensive and not something our suppliers advertise.
Thus the difference between mineral and paraffin oil is non-existent, though by calling an oil paraffin, it is perceived as a pure product because the word paraffin means “affinity for few”. There is a third hydrocarbon that can arise during oil refining – polycyclic aromatic hydrocarbons – or PAHs. These are very reactive molecules, e.g. benzene, and thus the USP and EP monographs both require the exact same test to assure they are non-detectable in the final product.
If oil is supposedly inert, why are we talking about quality issues?
One thing is clear: if an oil’s hydrocarbons are not fully saturated, whether alkenes, cycloalkenes or PAHs, they are susceptible to oxidation that can produce toxic peroxides in culture. A useful analogy is olive oil – it is monounsaturated and becomes oxidized over time leading to rancidity. Olive oil has a shelf life – eventually, it becomes oxidized and rancid.
In contrast, if mineral oil is fully saturated and lacks any readily oxidizable carbons, it is inert and requires enormous amounts of energy to become oxidized. To illustrate the stable nature of oil that is fully saturated, I’ve used the same batch of oil as a control for all of my studies on quality control, that was purchased in 2008. I even exposed one bottle from this lot to UV light for a week, and it has yet to develop peroxides and is nontoxic.
There are two classes of oil toxins: contaminants and reactive molecules called peroxides. Over 10 years ago my lab at the Mayo Clinic was the first to identify a contaminant in a batch of research grade mineral oil³. This oil was from a chemical supply company that does not test oil as rigorously as ART suppliers. We found high levels of Triton x-100, a detergent that was likely a carryover from the manufacturing process of the oil or the bottles containing the oil.
Since oil is, well, oil, it is very hydrophobic and will readily take up other hydrophobic molecules. This could potentially be a concern and requires that manufacturers have tight control of their supply chain to assure the oil is not exposed to high airborne concentrations of toxic volatile organic compounds (VOCs), and then, of course, good quality control testing to check for toxicity. The expectation is that thorough quality control testing will detect toxicity prior to shipping. One other approach to improve quality of oil is via washing – we showed that washing oil can reduce the concentration of TX-100 as well as the toxicity of peroxide-containing oil³.
Peroxides are far and away the most common cause of oil toxicity.4,5,6 Before an oil goes to the ART manufacturer, the refining plant treats the oil with high-pressure hydrogenation to effectively turn all double bonds, or alkenes, into single bond carbon chains (alkanes). Because of the homogeneous nature of the different hydrocarbons in oil, it is virtually impossible to isolate alkenes from alkanes, and the test for the presence of unsaturated alkenes is crude at best. Advanced analytic methods, such as FTIR, NMR, and GC-FID, offer promise in this area.
So what should we expect from suppliers of oil?
As mentioned before, we expect they have a robust process to assure the integrity of each batch of oil during shipping and handling7. Once they have a new lot in their facility, thorough testing of the batch is necessary to assure quality, testing that should be applied to all containers within the same lot.
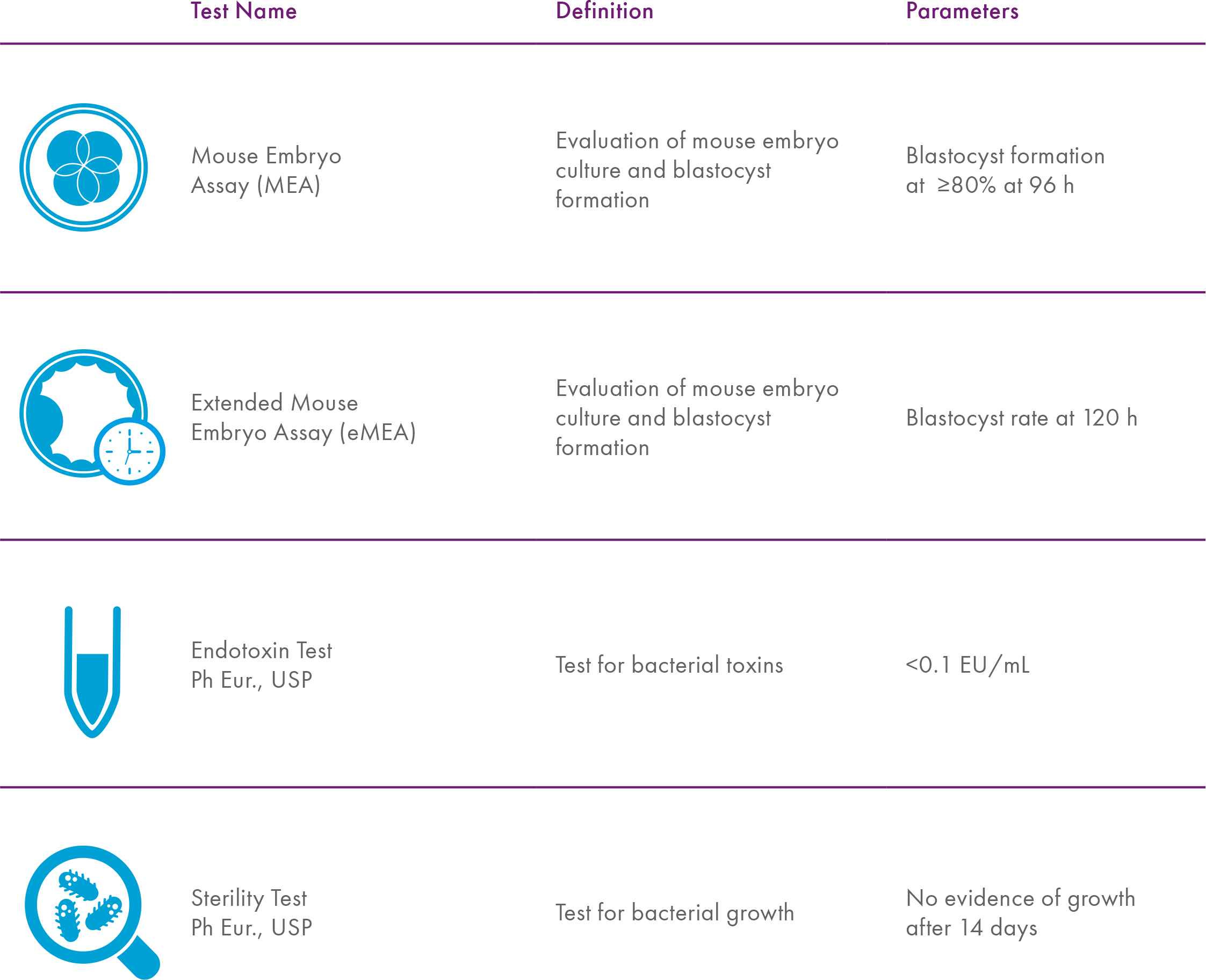
Thorough testing includes standard tests that are used for all products used in the IVF lab, like sterility, endotoxin levels, and a mouse embryo assay (MEA). Because of its nature, oil requires additional testing to assure quality. Through my research, we’ve learned that the standard MEA is not as sensitive to peroxides as human embryos and that by extending the assay by 24-48 hours, sensitivity improves8.
Additional tests that could provide improved toxicity sensitivity include blastocyst cell counts6, an ICM outgrowth assay9, using embryos from an outbred mouse strain10 or a timelapse QC assay11. The point is – you should expect more extensive bioassay testing.
Today we can expect our oil suppliers to test for peroxides as a minimal quality control to detect toxicity of oil and perform an enhanced bioassay. As end users, we can do our part by storing oil as per the manufacturer’s instructions.
Summary
While oil today is basically the same oil first used over 50 years ago, we can expect manufacturers to apply rigorous testing and quality control to assure we have high-quality oil for clinical use.
References
1. Morbeck DE, Leonard PH. Culture systems: Mineral oil overlay. Methods Mol Biol. 2012;912:325–31.
2. Elder K, Woodward B, Van Den Bergh M. Troubleshooting and Problem-Solving in the IVF Laboratory. Cambridge University Press. 2015(1): 37-38.
3. Morbeck DE, Khan Z, Barnidge DR, Walker DL. Washing mineral oil reduces contaminants and embryotoxicity. Fertil Steril. 2010;94(7):2747–52.
4. Otsuki J, Nagai Y, Chiba K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil Steril. 2007;88(3):741–3.
5. Otsuki J, Nagai Y, Chiba K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril. 2009;91(5):1745–9.
6. Hughes PM, Morbeck DE, Hudson SBA, Fredrickson JR, Walker DL, Coddington CC. Peroxides in mineral oil used for in vitro fertilization: Defining limits of standard quality control assays. J Assist Reprod Genet. 2010;27(2–3):87–92.
7. Morbeck DE. Importance of supply integrity for in vitro fertilization and embryo culture. Semin Reprod Med. 2012;30(3):182–90.
8. Ainsworth AJ, Fredrickson JR, Morbeck DE. Improved detection of mineral oil toxicity using an extended mouse embryo assay. J Assist Reprod Genet [Internet]. 2017;1–7.
9. Gada RP MD, Daftary GS MD, Walker DL MS, Lacey JM BA, Matern D MD PhD, Morbeck DE PhD. Potential of inner cell mass outgrowth and amino acid turnover as markers of quality in the in vitro fertilization laboratory. Fertil Steril 2012;98(4):0015-0282.
10. Khan Z, Morbeck DE, Walker DL, Fredrickson JR, Stewart EA, Coddington CC. Mouse embryos and in vitro stress: does mouse strain matter? Fertil Steril [Internet]. 2010;94(4, Supplement):S58.
11. Wolff HS, Fredrickson JR, Walker DL, Morbeck DE. Advances in quality control: mouse embryo morphokinetics are sensitive markers of in vitro stress. Hum Reprod [Internet]. 2013/04/19. 2013;28(7):1776–82.
 Dean E Morbeck PhD MBA, is Scientific Director at Fertility Associates in Auckland, New Zealand, and Honorary Lecturer in Obstetrics and Gynecology at the University of Auckland. After completing his PhD in Physiology at North Carolina State University in 1992, Dean undertook two Research Fellowship positions at the Mayo Clinic in Minnesota in 1993 and 1995. Since then he has been both teacher and mentor for numerous medical students, residents and fellows.
Dean E Morbeck PhD MBA, is Scientific Director at Fertility Associates in Auckland, New Zealand, and Honorary Lecturer in Obstetrics and Gynecology at the University of Auckland. After completing his PhD in Physiology at North Carolina State University in 1992, Dean undertook two Research Fellowship positions at the Mayo Clinic in Minnesota in 1993 and 1995. Since then he has been both teacher and mentor for numerous medical students, residents and fellows.
Dean has been working as Laboratory Director, Associate Professor and Consultant for various clinics across the USA since 1995 and in 2015 was awarded an MBA. Throughout his career, he has been a prolific contributor as member and chair of various professional and community societies, committees and services that include ASRM, Alpha, and ACE. He is also the author of many peer-reviewed articles, book chapters, abstracts, reports and publications.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada