Summary
The intracellular pH (pHi) of gametes and embryos is modulated by a number of systems and, in human oocytes and cleavage embryos, is around 7.1. In in vitro culture, the extracellular pH (pHe) of culture media is controlled within a typical range of 7.2 – 7.4. Tight control over this range to avoid pH deviation is prudent. An ideal pHe is, however, difficult to define due to variations between media formulations, culture conditions, as well as gamete specific or embryo stage specific preferences. In the IVF laboratory, pHe is generally controlled by adjusting the CO2 concentration of culture incubators, where it works in combination with media components, like bicarbonate, to reach equilibrium. However, due to varying conditions in individual laboratories, including media type, protein supplement and elevation, there is no single % CO2 concentration that will give the desired pHe or the best outcomes in all situations. When handling gametes and embryos outside the incubator, buffers such as HEPES or MOPS are typically used to control pHe.
Testing of pHe of culture media presents some technical difficulties but is recommended to verify culture conditions and aid in quality control and troubleshooting.
Introduction
The importance of pH in gamete and embryo biology is axiomatic and, in order to optimize embryo viability and reproductive outcomes, it is essential that pH be properly monitored and controlled. This applies equally (or more) to in vitro systems than in vivo. It is therefore essential that we appreciate the role of pH at various stages of the IVF process and understand how we can best control it.
Devised in 1923 by the Danish biochemist, Søren Peter Lauritz Sørensen, pH is a logarithmic scale expressing the ‘potential of hydrogen’ equal to -log10c where c is the concentration of hydrogen ions in moles per liter.
It ordinarily ranges between 1 and 10-14 hence the scale of 1-14 that we are familiar with. Importantly, as a result of the logarithmic nature of pH measurement, relatively “minor” changes in pH are actually large changes in hydrogen ion concentration.
In biological systems, cells have limited capacity for passive buffering of cytoplasmic pH and thus depend on a number of active transport mechanisms. In IVF systems, control of pH usually utilizes the bicarbonate buffer system (Fig. 1).
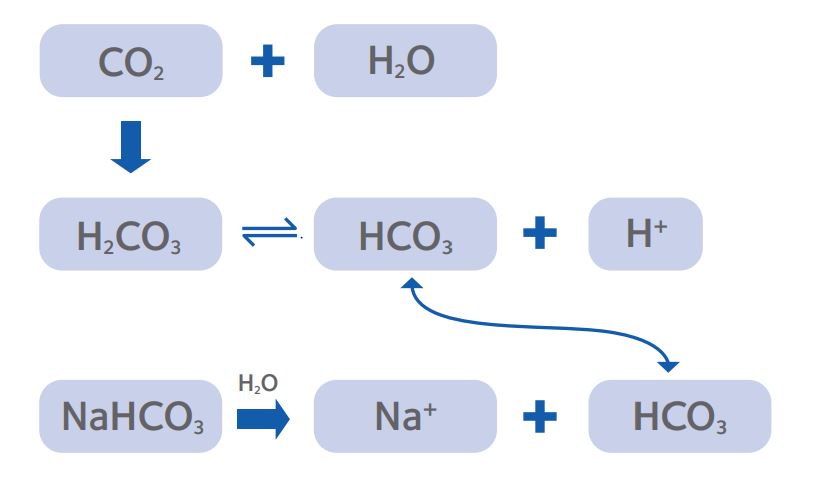
Figure 1: The CO2/ bicarbonate buffering system that is the
predominant method for establishing pHe of culture media.
When considering reproductive cells, mechanisms of pH regulation, such as the HCO3¯/Cl¯ exchanger and Na+ /H+ antiporter (Phillips et al, 2000), have been reported in human embryos. And whilst the focus has perhaps understandably been on embryos, the different requirements of various embryos stages, as well as the specific requirement of oocytes (denuded vs. cumulus enclosed) and spermatozoa, demand that we tailor conditions to meet the requirements of these different cells.
In this paper, we will look at pH in gametes and embryos and how it relates to development and function. Based on these findings, we will then consider how pH in our culture systems is manipulated. Finally, we will examine methods of testing pH to help validate proper and stable culture conditions.
pH in reproductive cells
The intracellular pH (pHi) of human oocytes and embryos is typically believed to be around 7.0-7.1 [Phillips et al, 2000], though a smaller study suggested it was around 7.3 (Dale et al, 1998), and is regulated by various mechanisms (for a review, see Swain 2010).
Interestingly, the capacity of oocytes to regulate pHi has been shown to be switched off between meiotic progression and fertilization, with the role conferred upon the supporting cumulus cells via gap junctions (FitzHarris and Baltz, 2006).
This exposes denuded metaphase II oocytes to fluctuations of pHi when pHe is poorly controlled, for example during procedures such as intracytoplasmic sperm injection (ICSI) or oocyte vitrification. Given that pHi impacts the cytoskeleton and meiotic spindle in rodents (Squirrel et al, 2001; Swearman et al, 2018), and similar impacts may be present in other mammalian oocytes, poorly controlled pHe has potential implications for rates of aneuploidy (Swain, 2019).
In preimplantation embryos, pH controls various cellular functions that influence embryo development. Squirrel and colleagues (Squirrel et al, 2001) reported disrupted localization of mitochondrial and actin filaments in mouse embryos when pHi was raised or lowered compared to controls at pH 7.2. Metabolism is also affected with increased glycolysis and reduced oxidative metabolism in response to rising pHi in hamster embryos (Lane et al, 2000). This is especially important in early cleavage embryo culture: in 1-cell mouse embryos, culture at an inappropriately low pHi resulted in reduced cell number in blastocysts, higher levels of apoptosis and reduced fetal growth (Zander-Fox et al, 2008). In contrast, later stages of embryos appear to regulate pHi more efficiently which is linked with the acquisition of tight cell junctions (Spindler et al, 2000). It is vital, however, to recognize that cryopreserved embryos may have a reduced ability to regulate pHi for 3-4 hours after warming/rehydration (Lane et al, 2000), so extra caution is advisable when handling and/or culturing embryos in these circumstances.
While oocytes and embryos favor mildly alkaline conditions, spermatozoa will function in the same conditions but thrive at higher pH. The pHi follows pHe in a quasi-linear relationship in human spermatozoa (Hamameh et al, 1996) but capacitation may rely on the selective activation of one major pHi regulator in the mouse at least (Zeng et al, 1996). Achikanu and colleagues (Achikanu et al, 2018) reported that higher pHe led to increased pHi and intracellular calcium concentration which resulted in hyperactivated motility; higher bicarbonate (HCO3¯) also drove increased sperm motility. Comparing various formulations of sperm washing media, de Rosa et al (2015) demonstrated how the composition impacted sperm function, again showing high pHe, as result of high HCO3¯ was beneficial. This begins to also demonstrate the difficulty in isolating an ideal pHe due to the independent actions of both CO2 and HCO3¯ on various cellular processes (Quinn & Wales 1974, Swain 2012, Swain 2011, Hentemann et al, 2011).
Control of pH in IVF systems
For routine culture, the pHe is determined by the equilibrium between the HCO3¯ in the medium and the level of CO2 in the incubator (as fig 1). Since most labs have moved away from making their own culture media, the concentration of HCO3¯ is effectively pre-set by the manufacturer, leaving the % CO2 as the parameter that can be manipulated by the laboratory to set pHe. Commercial media preparation is performed under tight controls and regulatory oversight, giving a high level of batch-to-batch consistency, and manufacturers test media for pH against a standard % CO2 which is then reported in the certificate of analysis provided with each new batch. Whilst a useful general guide to show that the medium composition is within specification, there are a number of factors to consider that might lead to variations in pHe in each user laboratory.
One factor affecting pHe is altitude: simply, the higher above sea level a laboratory is located, the higher the % CO2 in the gas mix to achieve the same pHe. For example, at sea level the standard barometric pressure is typically 101kPa (or 760mm Hg) but at 500, 1000m and 2000m above sea level, this drops to 96kPa, 90kPa and 81kPa respectively. Put another way, only 94%, 89% and 80% of the CO2 available at sea level is available at these altitudes even though the gas mix (by percentage) is the same (see https://baillielab.net/critical_care/air_pressure/).
It should be noted that the pHe will also depend on temperature, with pHe decreasing with increasing temperature, especially in zwitterionic buffered solutions, so stable control of this parameter is equally important.
Additionally, type and amount of protein supplement can affect pHe especially if added by the laboratory, both via the protein itself, but also via a dilution effect, essentially diluting the bicarbonate concentration v/v (Swain, 2012). Consideration of these factors is imperative in achieving the desired pHe and maintaining stability in the lab.
For quality control in the IVF laboratory, pHe can be thought of in 3 phases (Swain, 2010): equilibration, set point and stabilization (Fig. 2)
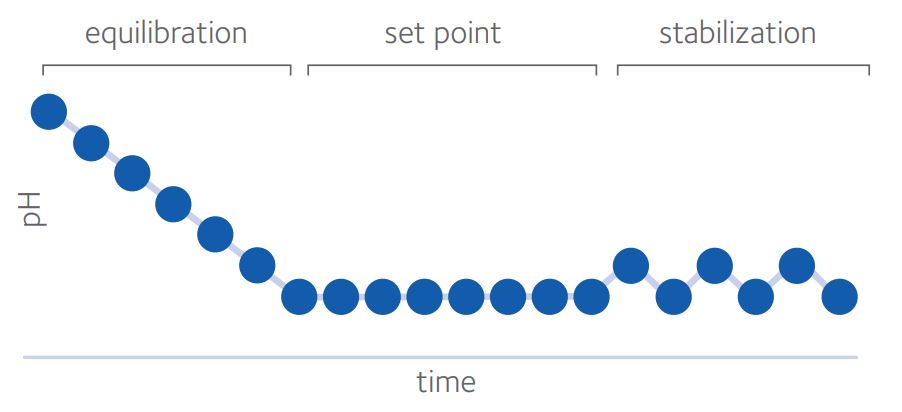
Figure 2: the three phases of pH in in vitro culture (from Swain, 2010)*
Time for complete equilibration will depend on volume of medium, surface area (influenced by drop size and/or well shape), oil overlay and type of dish used, as well as starting and ending pHe.
Sufficient time should be given for pH to reach equilibrium before exposing gametes or embryos. Steel and Conaghan (2008) suggested a minimum of 10 hours, whether using 50µl or 500µl under oil, though the ideal situation would be to validate equilibration times in each laboratory.
Set point is influenced primarily, but not exclusively, by CO2 and HCO3¯ with other components of the medium also having an impact. As already discussed, the pHi of oocytes and embryos is around 7.1. In embryo culture, it is crucial to avoid inducing acidosis and driving pHi below this figure and so the generally accepted lower limit for pHe is 7.2 to avoid acidification by intracellular metabolic processes. The upper limit is typically 7.4 since some reports (see Gatimel et al, 2020) suggest poorer outcomes at pHe >7.4. There may be some value in targeting pHe nearer 7.2 but it is impossible to give an ideal pHe as media composition also affects pHi (Swain 2010; Swain, 2012; Gatimel et al, 2020). Bearing in mind that a move from pH 7.2 to pH 7.4 represents a very significant 60% increase in hydrogen ions, it makes sense to attempt to stabilize pHe in our culture systems within a much narrower band. Various methods can be employed to aid in this stabilization.
Stabilization is the degree to which the pHe is unperturbed, taking precautions such as limiting the frequency of incubator doors being opened, using oil overlay or gassed isolettes or zwitterionic buffered media (with temperature considerations). Zwitterionic buffered media are modified media to provide a stable pHe under ambient atmosphere. These tend to have reduced HCO3¯ levels and the addition of a buffer, such as HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid) or MOPS (3-morpholinopropane-1-sulfonic acid), and allow longer handling times without pHe changes, which is especially useful for ICSI or embryo biopsy for example, (for review, see Swain & Pool, 2009; Will et al, 2011). A combination of buffers (eg HEPES + MOPS) offers possible benefits in terms of optimizing pKa (the acid dissociation constant equating to the pH where buffering capacity is maximal) and reducing individual concentrations and hence possible risk of toxicity (Will et al, 2011).
An additional consideration is to avoid pHe perturbation is during dish preparation or culture. Poor technique during preparation of dishes for microdrop culture or extended periods in a nonhumified environment without proper precautions can cause media evaporation (Swain et al, 2012). As a result, the solute concentration of the medium, including bicarbonate, increases. This results in an increase in pHe.
In summary, it is essential to select an appropriate set point for pHe, then ascertain what % CO2 is needed to generate this set point in the specific circumstances of each individual lab. Thereafter, one must implement adequate controls across the whole system to maintain the set point and minimize potential for fluctuations and/or drift. Allied to this, a proportionate and targeted system of testing pHe should be introduced.
Testing and internal quality control (IQC)
A recurring question is how and when the pHe of culture media should be tested. In the first instance, it is worth acknowledging the technical difficulties that many IVF labs encounter with implementing a robust methodology for pHe testing. These issues generally arise due to limited sample volume or other factors, but with emerging technology, can be addressed.
A reliance on accurate % CO2 has serious limitations but may have to be adopted as a proxy measure of pHe and incubator function if the latter cannot be reliably determined using a suitable pH meter. Whilst the use of incubator displays and fyrite should not be heavily relied upon (Pool, 2004), precertified gas mixes or appropriately calibrated external CO2 probes can provide some reassurances.
That said, provided it is performed well, direct pH testing should remain the gold standard. Accuracy and precision of pH measurement is reliant upon using an appropriate probe which is properly calibrated and used at the correct temperature (Swain, 2010). As well as solution temperature effects discussed above, there may also be electrode temperature effects so appropriate temperature compensation is needed; ideally pH measuring systems should be used and calibrated at the temperature a medium is used, usually 37°C. A very useful alternative is a hand-held blood gas analyzer (Gatimel et al, 2020) since this can test small volumes and has internal calibration, though systems should still be validated (Swain, 2013; Diaz de Pool et al, 2018; Cairo Consensus Group, 2020).
Though each has its pros and cons, none of these systems exactly match the culture system used in treatments and offer only snapshots of the pH. Systems are available, however, that provide real-time monitoring that can lend valuable insight into equipment function and impact of daily workflows or other lab environmental occurrences that may impact pHe. Systems such as SignipHy™, may offer a more robust approach.

SignipHy™ system for continuous real-time pH monitoring
A: sv2 sensor: single-use sensor measures pH continuously over 7 days
B: TrakPod: one per incubator; controls frequency of measurements
C: qc2 alignment tool: one per lab; part of QC testing for the system
D: TrakStation: data logging and user alarms for up to 8 TrakPods/incubators
* This article was published in Reprod Biomed Online, 21, Dr Jason E Swain, Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality, 6-16, Copyright Elsevier (2010).
References
Achikanu C, Pendekanti V, Teague R, Publicover S. Effects of pH manipulation, CatSper stimulation and Ca2+-store mobilization on [Ca2+]i and behaviour of human sperm. Hum. Reprod. 2018; 33: 1802–1811.
Cairo Consensus Group. ‘There is only one thing that is truly important in an IVF laboratory: everything’ Cairo Consensus Guidelines on IVF Culture Conditions. Reprod. Biomed. Online 2020; 40: 33-60.
Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998; 13:964–70.
De Rosa N, Pooley K, Kohut T, Dissing M, Campbell B, Kirkman-Brown J, Tomlinson M. Bicarbonate and pH in sperm washing buffers: effects on motility, sperm swimming speed and implications for ART. Fertility 2015, Human Fertility, 2015; 18:4, 291-302 P-8 Read Here
Diaz de Pool J, Van Den Berg S, Pilgram G, Ballieux B, Van Der Westerlaken L. Validation of the blood gas analyzer for pH measurements in IVF culture medium: prevent suboptimal culture conditions PLoS ONE 2018; 13(11): e0206707. https://doi.org/10.1371/journal. pone.0206707
FitzHarris G & Baltz J. Regulation of intracellular pH during growth and maturation in mammals. Reproduction 2009; 138: 619-627.
Gatimel N, Moreau J, Parinaud J, Léandri R Need for choosing the ideal pH value for IVF culture media J. Assist. Reprod. Genet. (2020) 37:1019–102
Hamamah S, Magnoux E, Royere D, Barthelemy C, Dacheux J-L, Gatti J-L. Regulators of sperm function: Internal pH of human spermatozoa: effect of ions, human follicular fluid and progesterone. Molec. Hum. Reprod., 1996: 2: 219–224,
Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril 2011; 95: 1291-4.
Lane M, Lyons E, Bavister B. Cryopreservation reduces the ability of hamster 2-cell embryos to regulate intracellular pH. Hum. Reprod. 2000; 15: 389-394.
Phillips K, Léveillé M-C, Claman P, Baltz J Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000; 15: 896–904.
Pool T. Optimizing pH in clinical embryology. Clin. Embryol. 2004; 7: 1–17.
Quinn P and Wales R. Fixation of carbon dioxide by preimplantation rabbit embryos in vitro. 1974. J Reprod Fertil. 1974; 36: 29-39.
Spindler R, Pukazhenthi B, Wildt D. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol. Reprod. Dev. 2000; 56: 163–171.
Squirrel J, Lane M, Bavister B. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol. Reprod 2001; 64: 1845-1854.
Steel T & Conaghan J. pH equilibration dynamics of culture medium under oil. Fertil. Steril. 2008; 89: suppl S27.
Swain J. Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality. Reprod Biomed Online 2010; 21 : 6-16.
Swain JE. Embryo culture and pH. Fertil Steril 2011; 95(8):e67;
Swain J. Is there an optimal pH for culture media used in clinical IVF? Hum. Reprod Update 2012; 18: 333-339.
Swain J. Comparison of three pH measuring devices within the IVF laboratory. Fert. Stert 2013; 100(3) S251.
Swain J. Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod Biomed Online. 2019; 39: 599-607.
Swain J & Pool T. New pH-buffering system for media utilized during gamete and embryo manipulations for assisted reproduction. Reprod Biomed Online 2009; 18: 799-810.
Swain J, Cabrera L, Xu X, Smith G. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod Biomed Online 2012; 24 : 142-147.
Swearman H, Koustas G, Knight E, Liperis G, Grupen C, Sjoblom C. pH: the silent variable significantly impacting meiotic spindle assembly in mouse oocytes. Reprod BioMed Online. 2018; 37:279–90.
Will M, Clark N, Swain J. Biological pH buffers in IVF: help or hindrance to success. J Assist Reprod Genet (2011) 28: 711-724.
Zander-Fox D, Mitchell M, Thompson J, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod. Biomed. Online 2010; 21: 219-229.
Zeng Y, Oberdorf J, Florman H. pH Regulation in Mouse Sperm: Identification of Na+ -, Cl+ -, and[formula]Dependent and Arylaminobenzoate-Dependent Regulatory Mechanisms and Characterization of Their Roles in Sperm Capacitation Developmental Biology 1996; 173: 510-520.
 Jason E. Swain, PhD, HCLD
Jason E. Swain, PhD, HCLD
Corporate Laboratory Director
CCRM IVF Laboratory Network in North America.
Dr Swain is an Adjunct Clinical Associate Professor in Obstetrics and Gynecology at Rutgers University and the University of Minnesota. He has published numerous peer-reviewed articles, edited and authored several book chapters and contributed to various other publications within the field of assisted reproduction. Dr. Swain serves on various committees for ASRM, ESHRE, PCRS and other organizations focused on assisted reproduction.
 David Morroll, PhD
David Morroll, PhD
Director of Clinical Support
CooperSurgical Fertility Solutions
Dr Morroll has worked as a Clinical Embryologist since 1986, training in Manchester, where he also completed his PhD studies. He has managed several laboratories in the UK and was previously Chair of the UK Association of Clinical Embryologists (ACE) and Association of Biomedical Andrologists (ABA). He has also been involved with several working groups, notably the HFEA Working Group on Safety and New Technologies and the Expert Group on Multiple Births after IVF. He joined ORIGIO a/s as Director of Embryology in November 2011 becoming for CooperSurgical in January 2019.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada