Commercial culture media are produced under the most stringent environmental conditions. Each time before a new batch is being released, the media are tested for quality under specific conditions; the Certificate of Analysis will show the pH of the media as tested in the production environment.
These conditions are unlikely to reflect entirely the individual conditions in your laboratory. In this article, I aim to highlight why identification and control of pH and its confounding factors in your own laboratory setting is so important.
pH is a logarithmic scale expressing the ‘potential of hydrogen’ equal to -log10c where c is the concentration of hydrogen ions in moles per liter. It is generally agreed that control of pH in IVF media is essential to maximize embryo viability and reproductive outcomes (Gatimel et al, 2020). This is especially true when gametes and embryos are particularly sensitive to the pH of their environment, specifically denuded oocytes and cryopreserved oocytes/embryos.
The first issue we encounter, however, is that we have only a guide to what the pH should be. The intracellular pH (pHi) of human oocytes and embryos is generally considered to be 7.0-7.1 [Phillips et al, 2000], so in IVF systems the extracellular pH (pHe) is typically in the range of 7.3 + 0.1.
Whilst setting pHe nearer 7.2 may be advantageous, we should also note that defining an ideal pHe is all but impossible because variations in media composition and inter-laboratory differences also affect pHi (Swain, 2010; Swain, 2012; Gatimel et al, 2020).
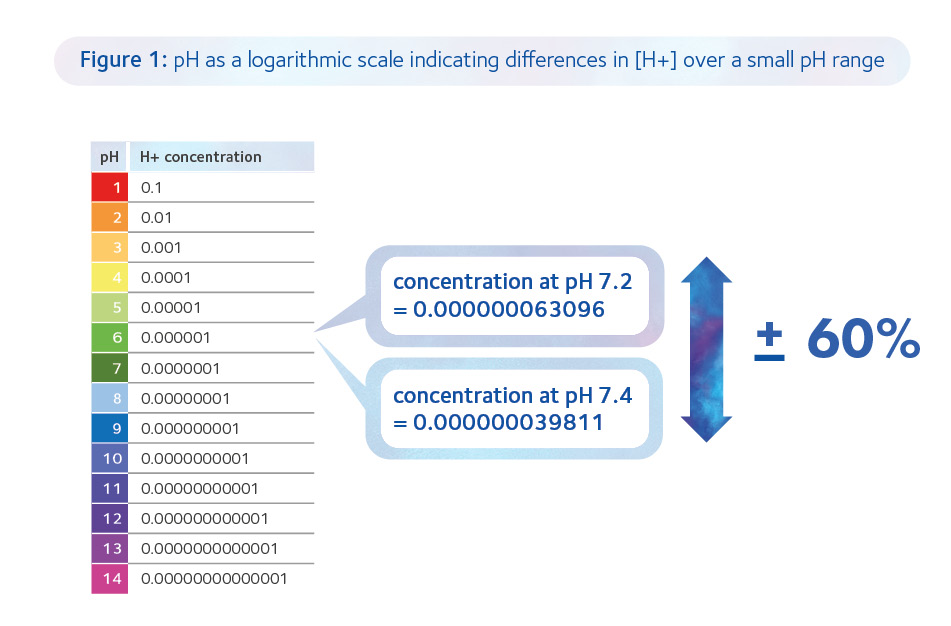
Hence, provided it is within the range of 7.2-7.4, the main emphasis for the lab should be to limit fluctuations in the pHe as small fluctuations represent significant changes in concentration of hydrogen ions (H+; Figure 1).
For culture media, pHe is determined primarily by the equilibrium between the bicarbonate (HCO3–) in the medium and the level of CO2 in the incubator, so with the concentration of HCO3– being set by the manufacturer, the laboratory is able to set pHe using the %CO2.
How is pH tested during production?
Commercial preparation of media is performed under tight controls, with an intensive system of audits by notified bodies that certify the process. Because of the rigorous oversight, there is the consistency of the raw products, the processes used, and the quality control applied.
When preparing products in bulk, the concentration of individual components can more easily be controlled so the concentration of HCO3– should be the same between batches and, hence, any possible fluctuations in pH minimized provided other factors are well controlled in the end user’s laboratory. Of course, testing of pH is one essential element of the package of comprehensive quality tests performed before the release of any product to customers. This involves testing pH when equilibrated with a standardized gas mix with a specific composition and % CO2 which will be cited on the certificate of analysis.
How should labs use the QC data and what additional in-house tests are needed?
The certificate of analysis shows the pH attained under very specific conditions in a tightly controlled environment. But, of course, this is not the ‘real world’ and so end-users should assess pH in their own system and focus on those variables that are specific to their lab. For example, we have highlighted % CO2 used but other factors include altitude and protein supplementation (for those media not pre-supplemented).
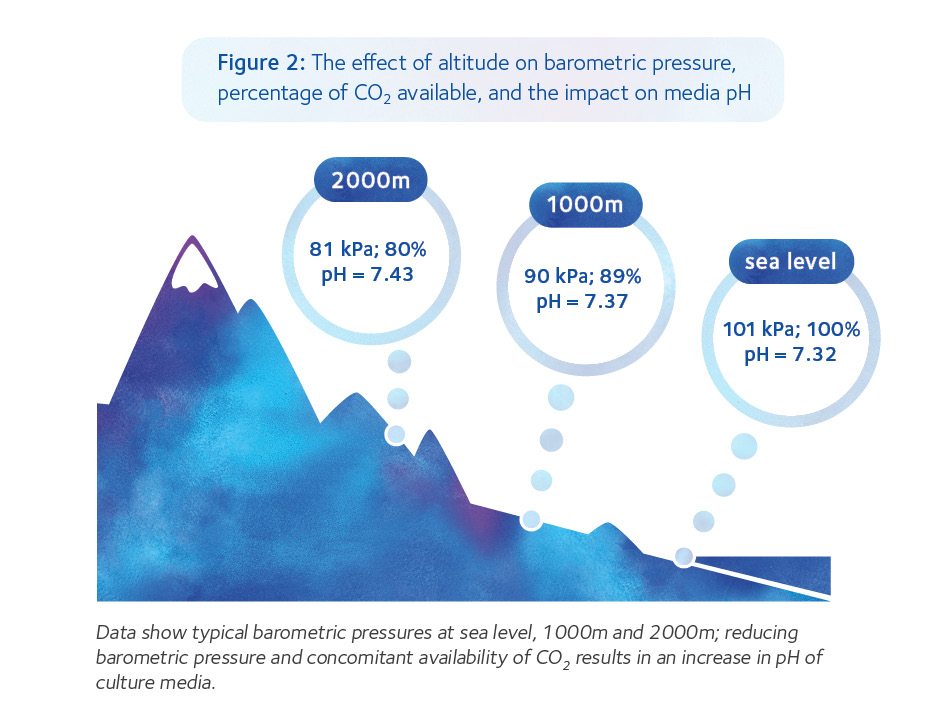
The higher above sea level a laboratory is located, the higher the % CO2 in the gas mix needed to achieve the same pHe. Due to decreasing barometric pressure, less CO2 is available for equilibration with increasing altitude if the gas mix (by percentage) is the same (Figure 2). Essentially, this means that it is not possible to give a single % CO2 that will deliver the target pH in all settings.
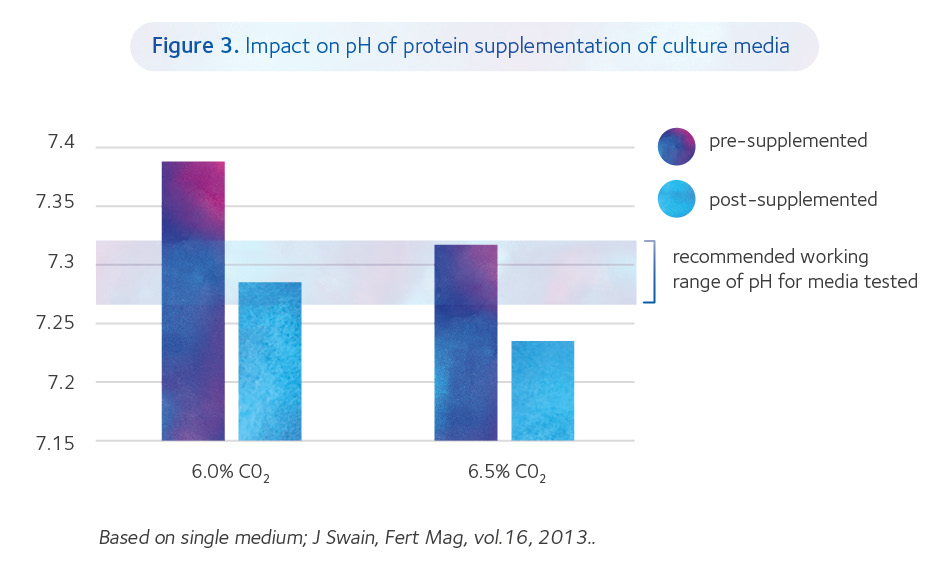
When adding protein to media, both the amount added and the type used can affect pHe especially if added by the laboratory (Figure 3), both via the protein itself, but also via a dilution effect, essentially diluting the bicarbonate concentration (Swain, 2012).
Another factor to highlight is the dependence of pH on temperature: pHe decreases with increasing temperature. For this reason, it is clearly important to control the temperature throughout the culture system and during the handling of gametes and embryos, but it is also crucial that pH testing be done at 37°C.
For additional information regarding pH testing see our webinar:
Revisiting pH in ART by Steve Levett, PhD, with Rachel Chin, MSc.
What other factors affect pH during culture?
We might think that checking our pH and setting the appropriate % CO2 for our laboratory set up, then ensuring this is all maintained, would be sufficient. There may, however, be other dangers lurking elsewhere in our system!
Jason Swain describes the three phases of control of pHe in the IVF laboratory (Swain, 2010): equilibration, setpoint, and stabilization. In pH testing, we tend only to determine compliance with our targeted set point. Whilst full equilibration depends on the volume of medium, surface area (influenced drop size and/or well shape), oil overlay, and type of dish used, these factors can also influence the actual pHe to which oocytes and embryos are exposed during normal working, not least because they also determine the impact of perturbations of pHe caused during routine handling.
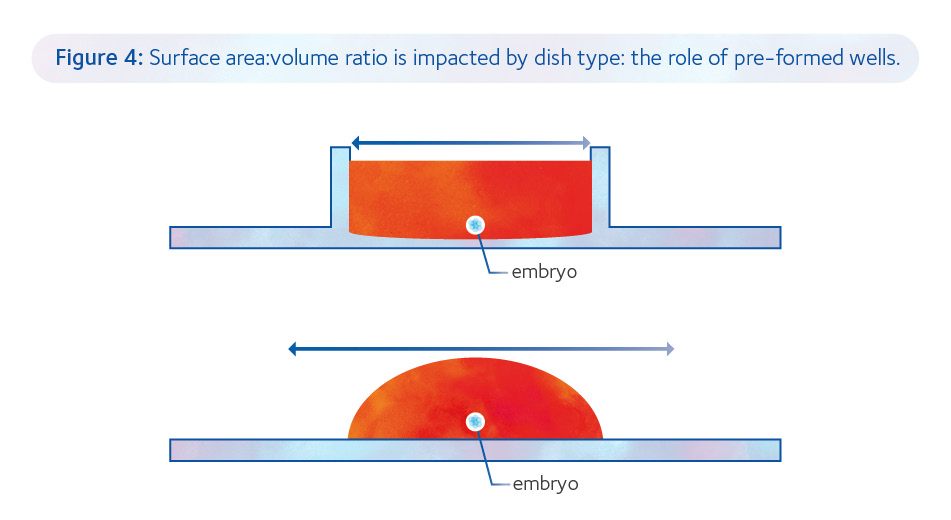
For example, let’s consider the volume of micro drops. It is clear that poor technique during the preparation of dishes can cause media evaporation (Swain et al, 2012), resulting in increased solute concentrations, including bicarbonate, increases with a concomitant increase in pHe. Larger drop sizes were shown to mitigate this risk but osmolality changes during culture can be further minimized by using dishes with pre-formed wells as the surface area:volume ratio is decreased (Figure 4).
The volume of oil overlays also plays a role: in SAFESens testing vials, pH increased after 7 days by 0.09 when overlaying test medium with 35µl rather than 50µl. The key message is to use the maximum volume of oil for the type of dish being used, taking care not to cause spillages that might effectively seal the rim of the dish and impair gaseous exchange.
It is often impossible to test pH in drops in the dishes used for culture because testing systems often require larger volumes. This needs to be borne in mind when determining the impact of pH on outcomes.
Take-Home Messages
- The viability of gametes and embryos is impacted by pH and especially at certain stages such as denuded oocytes.
- Though useful, the pH testing by the manufacturer is done under rigorously controlled conditions that are unlikely to reflect conditions in labs.
- Hence, labs must take account of their specific circumstances and test appropriately.
- Care is needed to control confounding factors, such as temperature and volume/type of oil, as well as setting up of the culture system.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada