This webinar will be a step-by-step guide to ICSI, with a critical appraisal of the technique’s efficacy. It will include a description of indications for ICSI, technical aspects of sperm preparation (including sperm from epididymis or testis) and preparation of oocytes before injection, oocyte and sperm selection as well as the injection itself.
The set-up and optimization of the ICSI equipment and procedure will be discussed. The webinar will also focus on how to set up a training program and use KPI’s to monitor quality on the ICSI program.
In this webinar, CooperSurgical Global Training Manager, Martine Nijs provides a step-by-step guide to Intra-Cytoplasmic Sperm Injection (ICSI).
She includes a critical appraisal of the technique’s efficacy and covers a description of indications for ICSI, technical aspects of sperm preparation (including sperm from epididymis or testes) and preparation of oocytes before injection, oocyte and sperm selection as well as the injection itself.
She also discusses the set-up and optimization of the ICSI equipment as well as the procedure itself. The webinar also focuses on how to set up a training program and use KPIs to monitor quality on the ICSI program.
Following a brief introduction on the development of ICSI, she discusses the indications and outcomes for it. She then looks at the fertilization process and continues with the practical aspects of ICSI in the laboratory.
As part of this, she spends a little time describing sperm prep, oocyte prep, real-time selection of spermatozoa, ICSI itself and all lab actions post-ICSI. She then focuses on how to optimize ICSI outcomes, followed by a brief glance at the future possibilities, before offering up a summary.
Introduction
As we all know, the efficiency of in vitro fertilization is limited by the functional and kinetic properties of the spermatozoa. In the 90s, different manipulation techniques were used to increase the chance of a spermatozoa to reach the oolemma and fertilize it naturally.
In a first approach, called zona drilling or zona partial dissection, embryologists used an acid, an enzyme or a needle to partially thin or open the zona pellucida. A second approach was to aspirate multiple spermatozoa from a sperm drop with a large pipette and inject them in the perivitelline space. The latter technique was named subzonal injection.
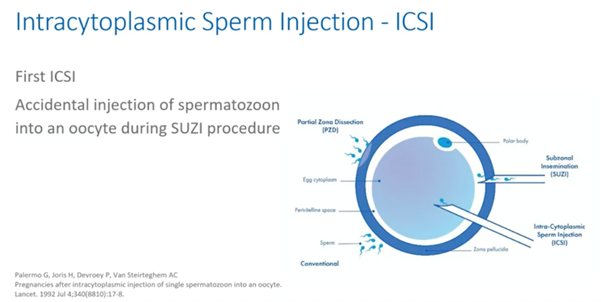
Both techniques resulted in an increased fertilization rate but also in a very high polyspermia rate. It was during one of these SUZI procedures that, by accident, a sperm cell was injected into the oocyte. This oocyte showed to be normally fertilized the next day as published by the group of Palermo and colleagues from Brussels.
As defined by this group, ICSI is a procedure that entails the deposition of a single spermatozoon directly into the cytoplasm of the oocyte, thus bypassing the zona pellucida and the oolemma.
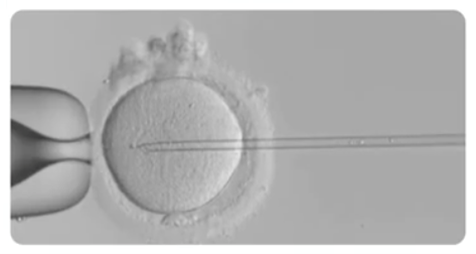
It’s obvious that ICSI is a high precision procedure through which an oocyte can be fertilized independently of the morphology and or motility of a single spermatozoon injected.
Indications and outcomes for ICSI
ICSI was an immense breakthrough for the treatment of patients suffering from male factor infertility. Patients suffering from severe oligozoospermia and later even azoospermia, by retrieving sperm cells from the epididymis or testes were offered a chance to father a child. Since then, ICSI usage for non-male factor is increasing globally.
ICSI is being used for unexplained infertility, poor quality oocyte, low oocyte yield, advanced maternal age, prior fertilization failure with conventional IVF, pre-implantation genetic testing (PGT), fertilization after IVM and fertilization of cryopreserved oocytes and oocyte donation cycles.
Some clinics also opt for ICSI for all cases. The data from Europe as presented by the ESHRE EIM Consortium in 2020, show again, the ongoing trend toward more ICSI usage – accounting for more than 70% of the cycles performed.
So, are we doing a good job? As presented at the ESHRE meeting 2020, the ICMART data from the year 2016 includes global data for non-donor cycles and does not include data from China. They clearly show a delivery rate of 19.2% per aspiration, both for ICSI and IVF.
The next question to ask is, is ICSI safe? Multiple studies have been published on the health of the children born. Unfortunately, there are many pitfalls and inconsistencies in these study designs but globally, the data provides reassuring evidence regarding the health of ICSI children for male indications.
The data shows that there is a higher risk of pre-term birth and small-for-gestational age as well as an increased risk for congenital malformations, chromosome aberrations and less favourable cardiometabolic outcomes. These risks are at least partially related to parental infertility and can also result in transgenerational inheritance of the infertility. So, sons inheriting poor sperm quality from their father, for example. Studies on the long-term follow-up of ICSI children (thus adults) are so far scarce.
ICSI or IVF?
Who benefits from ICSI in the non-male factor group?
The ASRM Committee analyzed data on usage of ICSI in the non-male factor group. It found that ICSI may be of benefit for select patients with preimplantation genetic testing for monogenic disease or previously cryopreserved oocytes.
For all other indications such as unexplained infertility, poor quality oocyte, low oocyte yields, advanced maternal age and fertilization after IVM, ICSI didn’t seem to result in better outcomes. The exception for this was among patients with previous failed, or no fertilization cycles. Here it was found it could improve the fertilization rate.
ICSI and the Process of Fertilization
How can an oocyte be fertilized by ICSI?
Let’s remind ourselves of just what a specialized and fascinating mechanism the fertilization process is. Freshly ejaculated sperm are unable or poorly-able to fertilize. Rather, they must first undergo a series of changes known collectively as capacitation.
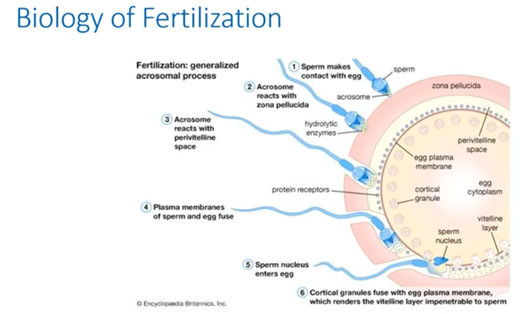
Capacitation is also associated with the removal of adherent seminal plasma proteins and reorganization of plasma membrane lipids and proteins.
The sperm head carries the acrosome, which is a lysosome packed with zona-digesting enzymes. After the acrosome reaction, the enzymes are released and the zona pellucida is dissolved.
The acrosome will react with the PVS and the sperm and egg membrane will fuse. Upon binding with the sperm, the egg will then rapidly undergo a number of metabolic and physical changes. We collectively call this egg activation.
Prominent effects include a rise in intracellular concentration of calcium, completion of second meiotic division and cortical reaction. The latter is a massive exocytosis of cortical granules seen shortly after sperm oocyte fusion.
These proteases alter the structure of the zona pellucida and it will be impenetrable for other sperm cells. The male pronucleus will be formed and DNA will decondense. The male pronucleus will then migrate to the female one and they will fuse to form the diploid nucleus. The second polar body is then extruded.
In ICSI, the initial steps of fertilization are bypassed. Namely the movement to, the binding to and the penetration of the zona pellucida as well as the fusion with the oolemma. The following biological processes still take place however and these include:
- The rise in calcium in ooplasm
- Oocyte activation
- Formation of the male pronucleus and DNA decondensation
- Migration of the male pronucleus, pronuclei fusion and formation of the diploid nucleus
- Extrusion of the second polar body
Two steps in the ICSI process are crucial. The first is the aspiration of the cytoplasm into the ICSI pipette to break the oolemma and induce oocyte activation. The second one is the immobilization of the sperm so that the sperm can release oocyte-activating factors into the ooplasm. This will also induce a rise in calcium.
Sperm Preparation for ICSI
Sperm preparation techniques should:
- Remove all seminal fluids
- Remove all non-sperm cells
- Isolate morphologically normal and motile sperm
- Support capacitation
- Reduce DNA fragmentation
Sperm preparation techniques should isolate and select viable spermatozoa with:
- Intact functional and genetic properties
- Normal morphology
- Minimal DNA damage
- Intact cell membranes with functional binding properties
There are a range of sperm preparation techniques available. I don’t have the time to address them all in detail, so I’ll focus on the three most simple and widely used. These include wash and concentration, swim up and density gradient.
Sperm preparation: wash and concentrate
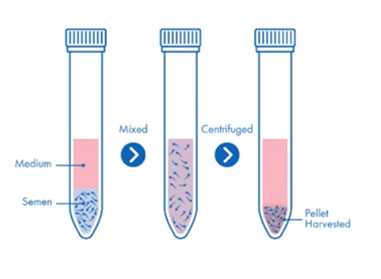
Diluting semen with cultural media and centrifuging, is by far the simplest method. Because it compacts the healthy sperm into a pellet with non-sperm cells and poor quality and dead sperm, there is a real risk of reactive oxygen species damaging the healthy sperm.
This, together with the fact that there is no selective process to refine the population of sperm, will mean that the wash and concentration technique should be a last resort. Generally, it’s not recommended.
The main features of the wash and concentrate technique include:
- Centrifugation removes seminal plasma and concentrates the specimen into a small volume
- It’s simple, fast and inexpensive
- It does not remove non-viable sperm cells and so increases the risk of formation of reactive oxygen species
- Pellet may be resuspended in fresh media or added to media drops in ICSI dish
Sperm preparation: swim-up
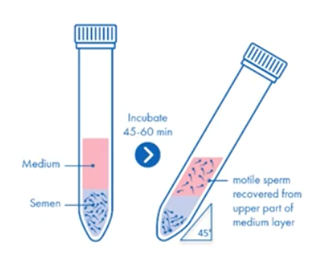
The swim-up and other sperm migration techniques rely on motility so are best for samples of generally high quality, but this is not always the case in ICSI samples.
As for the washing concentration, it generally isn’t recommended that sperm samples are washed and then a swim up performed. Rather, medium is layered over whole semen and the collected sperm is washed.
The main features of the swim-up technique include:
- Sperm are selected by their ability to swim out of seminal plasma and into overlying medium later
- It’s simple, fast and inexpensive
- Isolates motile and morphologically normal sperm
- Reduces ROS, oxidative stress and DNA fragmentation
- Lower yield and efficiency
Sperm preparation: density gradient
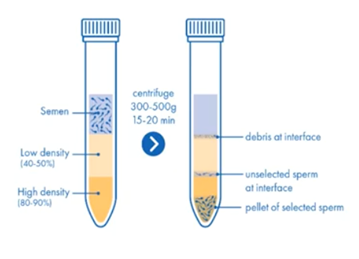
Density gradient separates cells by their density and will result in an optimal yield. The amount and volume of the layers can be adapted according to the initial analysis.
The main features of the density gradient technique include:
- It’s more labour intensive and therefore costs more
- Provides a clean fraction of highly motile spermatozoa
- The formation of reactive oxygen species is significantly reduced
- It’s used for patients who test positive for potentially infectious diseases
Choosing a Sperm Preparation Method
The choice of sperm preparation methods depends on the characteristic of the semen sample. If concentration and motility are within the normal ranges, the direct swim-up technique can be used.
For significantly OAT samples, which is mostly the case in ICSI, density gradient configuration is preferred because it leads to a higher density recovery rate of motile sperm cells compared to the swim-up technique.
Density gradient centrifugation can be modified to address the issues of each individual specimen. It’s the method of choice for sperm preparation in the majority of ART and andrology laboratories.
Sperm preparation: surgical sperm retrieval from testis
In case of testicular biopsies, the spermatozoa need to be isolated from the testicular tubule. This can be done in a mechanical way by milking with needles, mixing through a tissue grinder or slitting with sterile glass slides.
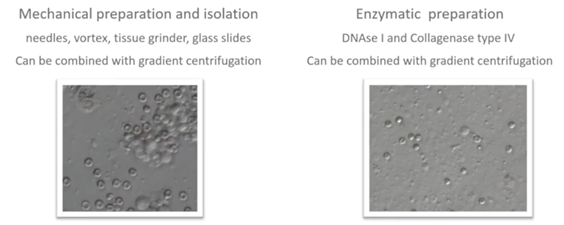
The tubular material can also be digested with enzymes like DNAse I and Collagenase type IV. This method will also result in the lysis of excessive red blood cells. It’s very useful in case of non-obstructive azoospermia and in addition to mechanical methods.
If a good number of spermatozoa are present, we can centrifuge the cell suspension with culture media or we can even apply a mini density gradient centrifugation. The supernatant is removed, and the pellets are resuspended in fresh culture media.
Denudation of COC’s
The removal of the cumulus complex and corona radiata surrounding the oocyte, is necessary to easily fix the oocyte on the holding pipette. This is also to have a good view during the injection of the zona pellucida, the oolemma and the ooplasm and of course to check the quality and maturity of the oocyte.
We generally use the enzyme, hyaluronidase. This is found in high concentration in the acrosome of most mammalian spermatozoa. This can be recombinant, bovine or ovine of origin.
I will describe a typical denudation by using Cumulase as an example.
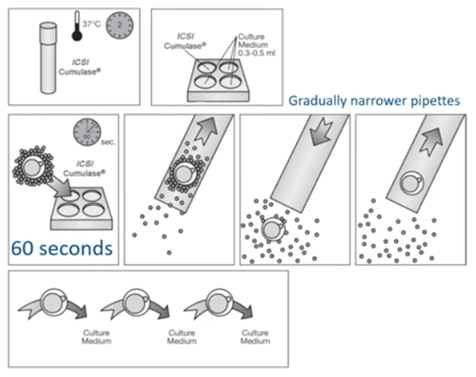
First, we have to prepare all our denudation pipettes. Then, we pre-equilibrate the medium containing the enzyme for two hours at 37 degrees C. This is of course according to the Instruction for Use (IFU) of the supplier.
When the two hours have passed, we will take 0.3 to 0.5ml and transfer it into a multi-well dish. One of several oocyte cumulus complexes are placed in the first well with a large pipette. After 60 seconds, the cumulus-oocyte-complexes are gently aspirated up and down in a denudation pipette and transferred to a second well with buffered medium. The remaining cumulative cells are removed by gently aspirating the oocytes up and down in the denudation pipette with the smaller diameter.
Once the cells are removed, we wash the oocyte thoroughly by transferring them between several wells of culture medium to remove all traces of the enzyme. The oocytes are then transferred to the culture dish and placed in individual microdrops, covered with pre-equilibrated liquid oil and cultured until ICSI is performed.
Care needs to be taken that temperature, osmolarity and pH are stable throughout this first manipulation step.
Gentle pipetting is performed to avoid mechanical stress on the oocytes. Washing of oocytes after denudation to remove all traces of enzyme is key. Of course, witnessing of the ID should be ensured during all denudation and culture steps.
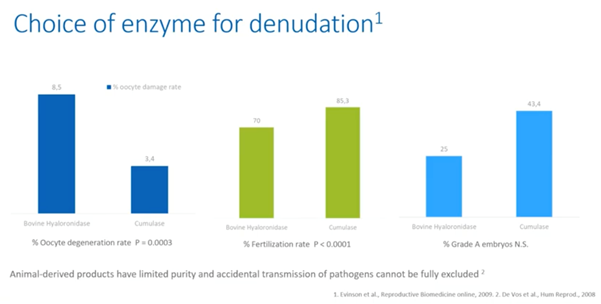
Choice of a denudation enzyme
On the one hand, animal-derived products have limited purity and therefore, accidental transmission of pathogens cannot be fully excluded. On the other hand, studies show that the use of recombinant enzyme Cumulase for denudation resulted in lower oocyte degeneration rates and better embryo qualities.
After denudation, we can score maturity and quality of the oocytes.
ICSI is generally performed on MII oocytes. ICSI on in vitro matured MI-MII oocytes can be done, but will result in a reduced fertilization rate, high aneuploidy rate, low pregnancy rates and very low ongoing pregnancy rates.
As for the quality of the oocyte, the Istanbul Consensus and the work of Rubini and colleagues lists the following abnormalities as a negative influence on the outcomes and therefore should not be inseminated.

The giant oocyte, as you can see in the picture at the top left, is a possible source of dygenic triploidy.
The abnormal first large (but not fragmented) polar body is pictured at the top right. There is a risk of aneuploidy in this oocyte. Oocytes with large perivitelline space increase cytoplasmic granularity and large vacuoles persisting past syngamy. These shouldn’t be inseminated because they will result in poor outcomes.
Oocytes with smooth endoplasmic reticulum aggregates or discs can result in normal embryos and can be injected as demonstrated by the work of Simopoulou.
Equipment and pipettes
Now let’s look at the hardware, the equipment and pipettes and so forth that we need for our ICSI.
Here I’ve listed the minimal equipment that needs to be in place to perform high quality ICSI procedures.
- Flow cabinet type II
- Stereomicroscope with heated stage
- Incubators – non-gassed and CO2 gassed
- Inverted microscope with micro manipulators
- Two injectors – this could be air or oil
- Anti-vibration table
- Low energy laser system
- Screen
- Stable heated stage and/or a heated crib
- No direct airflow above the micromanipulators
- Adjustable chairs because ergonomics are very important for a healthy working position
As for the micro manipulation pipettes, we need high quality. These are sterile and tested and defined for use in IVF. The typical size of the holding pipette ranges from 100 to 120 micrometers for the outer diameter. An ICSI pipette generally has a controlled inner diameter of 4 to 6 micrometers.
A new pipette must be used for each patient.
A witnessing system also needs to be in place. This is to avoid adverse events and ensure traceability of all process steps and batch and stock controls.
The ICSI procedure – an in-depth look
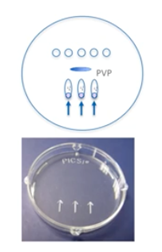
We’ll use a standard 9mm ICSI dish that’s designed for IVF. Do not use the lid of the dish because it could be toxic. Then we need oocyte, sperm, SpermSlow and/or PVP drops. These should be easily distinguished and spaced to avoid merging and mixing.
The layout should help keep track of which oocytes have been injected so the drops should be numbered. They shouldn’t be too close to the dish edge because this interferes with the tools. Equilibrate the dish at 37 degrees for a minimum of 30 minutes in a non-gassed incubator before starting the ICSI procedure.
Here are two examples of a dish layout but there are many variations of this.
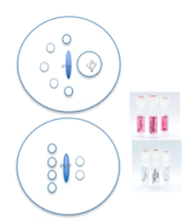
It’s best is to place drops of 10 to 20 microliters of HEPES medium and/or MOPS-buffered medium supplemented with HSA, as centrally as possible. There should be one drop per oocyte.
We add the PVP drops and extra drops of the HEPES and/or MOPS-buffered medium for sperm collection and/or for washing of pipettes.
We overlay with six ml of pre-warmed mineral oil for embryo culture. Then we’ll equilibrate those dishes for a minimum of 30 minutes in a non-gassed incubator.
A SpermSlow dish has a slightly different design. Again, we will use 10 to 20 microliters of HEPES and/or MOPS-buffered medium drops for our oocytes. We will, however, place a PVP drop as well as a SpermSlow drop.
During ICSI, we will move from the SpermSlow into the PVP so they shouldn’t be too far away from each other. Again, we will make an overlay with oil and equilibrate them in a non-gassed incubator for at least 30 minutes.
The PICSI dish has pre-loaded dried hyaluronan microdots for advanced sperm selection. We rehydrate by adding five microliters of buffered media, pulling into an elongated teardrop shape. Take care not to touch the microdots.
Quickly cover with oil to avoid evaporation then equilibrate for about 10 minutes in a non-gassed incubator.
When ICSI commences, we then add the sperm at the tip of the drop furthest from the microdot. This is so they can swim toward the hyaluronan drop.
For epidydimal and testicular sperm samples, an extra drop with the sample is placed under oil and sperm cells are collected individually from this prepped sample. They are then brought into a collection drop before performing the ICSI itself.
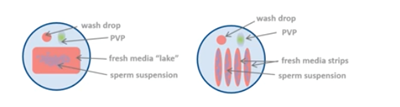
If you have good numbers and motility, you can use a standard dish layout. If numbers and motility are poor, plate out to allow motile sperm to swim out into a clean medium.
Before we start, we’ll clean the eye pieces, the manipulators and the holding system. We’ll also check the laser alignment and function and the temperature on the heated stage. We will install holding an ICSI pipette and we will check our injectors.
The tip of the ICSI pipette should be orientated slightly downwards so we can immobilize easily.
Next, we’ll label the ICSI dish with the patient’s name and number oocyte drops. We’ll move the gametes into the dish and of course, here we need witnessing so there isn’t a mix-up of gametes.
We’ll bring the ICSI dish to the inversion microscope and prime and test the ICSI and holding pipette in the extra wash drop by aspirating some medium in and out of the pipettes.
The ICSI technique
The ICSI technique consists of three steps.
Step one
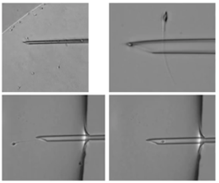
The first step is selecting the spermatozoon and we’ll go into detail about how to do this a little later.
We then immobilize the spermatozoon. We do this by touching the flagellum of the spermatozoa, fixing it between the pipette and the ICSI dish.
Next, align the spermatozoon with the pipette and then aspirate it. Stabilize the spermatozoon in the tip of the ICSI pipette and then move to the oocyte drop.
Step two
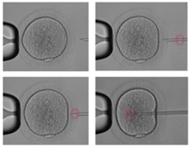
In the oocyte drop, orientate and hold the oocyte with the holding pipette. Bring the pipette into the equatorial plane. Focus on the oolemma and bring the spermatozoon to the tip of the pipette. Slowly push the ICSI pipette through the zona pellucida in the oocyte.
Step three
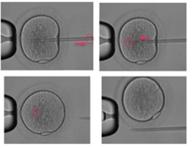
The third step consists of aspirating the cytoplasm and then re-injecting it plus the spermatozoon. Then slowly retract the pipette from the oocyte, staying in a nice horizontal plane. Stop aspirating the oocyte with the holding pipette, and then remove it from the holding pipette.
Post ICSI – wash and culture
Following injection, oocytes should be washed and returned to culture in a new equilibrated labelled dish. The oocytes should be washed through drops of culture media and incubated overnight in a gassed incubator.
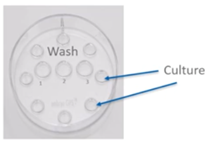
It’s important to witness the move from the ICSI dish to the culture dish and note all details on the ICSI procedure and the oocyte quality in the patient’s dossier.
Real-time selection of spermatozoa
During the ICSI procedure, the real-time selection of spermatozoa is an important step.
To select viable from dead spermatozoa in a pool of immotile spermatozoa, we can use the following methods.
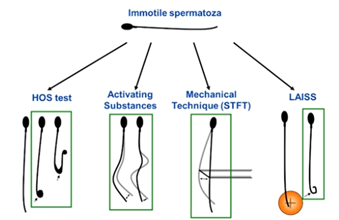
We can perform a viability test on each individual sperm cell just prior to the ICSI. There is, for example, the HOS Test (the hypoosmotic swelling test) where live spermatozoa will curl their flagellum when placed in hypoosmotic solution.
We can also check the viability by touching the tail with the pipette for reaction of the flagellum – this is the mechanical method.
A third method is to laser the tail at the end of the flagellum so you’re checking again for a reaction – yes or no.
The fourth method is exposing the spermatozoa to a range of substances. These include methylxanthines (pentoxifylline or theophylline), caffeine or myoinositol. The pentoxifylline is the most widely used and it will stimulate motility.
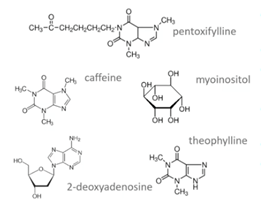
It is of course key that after incubation, there’s a washing step and that this substance is not injected as such into the oocyte.
We can also do a real time selection for morphology. At normal magnification, we have to select the spermatozoa under morphological appearance. There is a correlation between the head shape and aneuploidy. The majority of large headed spermatozoa are diploid. 25% of spermatozoa with amorphous heads, round heads and elongated heads have chromosomal abnormalities.
Globozoospermia have high DNA fragmentation and no acrosome. This means artificial activation could be an option for these samples. Lastly, the presence of cytoplasmic remnants is a sign of nuclear immaturity.
Another approach is the IMSI – the intracytoplasmic morphologically selected sperm injection technique at high magnification (6,300x).
Here, we use advanced optics to examine the ultrastructure of sperm and look for the presence of vacuoles as suggested by Setti and colleagues.
We can use this method to improve pregnancy rates and reduce miscarriage rate.
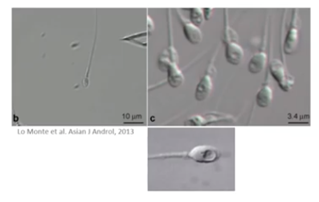
A Cochrane review of randomized controlled trials does not support the clinical use of IMSI. It found evidence that suggests IMSI improves clinical pregnancy to be of very low quality.
A later Cochrane review suggested that IMSI may help sperm selection and be effective in overcoming later paternal effects such as blastocyst formation and implantation.
Another option for individual sperm selection is hyaluronan binding. If sperm cells bind to hyaluronan, it will reflect cellular maturity, reduced risk for DNA fragmentation and aneuploidy and increased chromatin integrity.
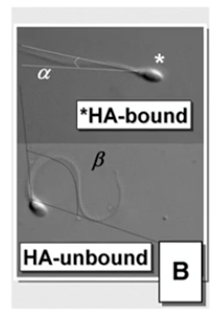
A large study in the UK showed a significant reduction in miscarriages when using PICSI dish for sperm selection, particularly in older women. The live birth rate was slightly higher but not statistically significant. For individual couples, lowering the risk of miscarriage is very relevant, however. It should be noted that the study was performed on a broad population and sperm selection may not be relevant for all couples.
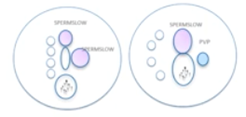
Maturity and DNA integrity – hyaluronan binding
There are two systems for hyaluronan binding. One is the PICSI dish which you can see on the left.

As I discussed before, once the plaque of the PICSI dish is rehydrated, sperm can be added and allowed to swim across that plaque. Those expressing receptors to hyaluronan may bind to the surface of the dish in the plaque and then be aspirated into an ICSI pipette.
The other system is SpermSlow which is shown on the right in the image above. Sperm are introduced into a drop of the hyaluronan-rich medium. If expressing receptors, they will get trapped in the gel matrix and lose progressive motility and shudder on the spot. We then can pick those spermatozoa, go to the PVP, immobilize and use them for injection.
Fertilization after ICSI
The day after our ICSI, we can check fertilization by the presence of 2PNs and two polar bodies in the manipulated oocytes. Roughly 1% will show 1PN oocytes. Preferably, these 1PNs shouldn’t be transferred since they can be haploid because of parthenogenesis or they could show asynchronous PN formation or have PN fusion which means they’re diploid.
We do know from studies that if they are diploid, they have a higher aneuploidy rate than normal 2PNs.
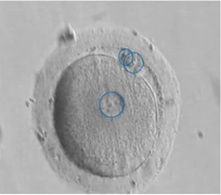
Failed fertilization
Failed fertilization can also occur, and in less than 1% there is a total failure to fertilize. It’s really difficult to pinpoint the exact cause because it could be oocyte and/or sperm related.
Optimal conditions for ICSI
Now I’ll quickly touch on some factors which can influence the quality of your ICSI and its outcomes.
Multiple factors will influence each step of the fertilization and culture process in our labs. It’s our professional task to ensure that the stress on the gametes and the embryos is minimalized during these different steps.
Temperature
Temperature is a key one and this needs to be stable between the limits of 36.7 and 37 degrees. This is to avoid instability of the meiotic spindle in the oocyte. A uniformly heated stage like the Thermosafe for example is mandatory.
Remember to check if there’s an air gap between the bottom of the dish and the heated stage. Be careful if the temperature differs between the culture and ICSI dishes.
Management of equipment and culture systems
Equipment and culture systems need to be validated, maintained and monitored rigorously to avoid any sudden stress on gametes and embryos.
Optimal ICSI technique
Now let’s look at some technical steps that could optimize your ICSI procedure and outcomes.
It is for example, important to have the Polar Body at six or 12 o’clock – it’s your choice. The Polar Body is not a good indicator for the meiotic spindles so you can put it at six or 12 o’clock without interfering with the outcomes.
The opening of the ICSI pipette should be either towards or away from the Polar Body. Preferably, it should be away from it in case the meiotic spindle is there. There’s no proof of better or worse results from this, however.
Should you use a large or small pipette for injection? We know that larger pipettes may harm cytoskeleton which in turn might have consequences on blastocyst formation.
Should we use spiked or non-spiked pipettes? Again, this is your choice but be aware that the spiked pipette can damage the opposite oolemma of the oocyte.
What about rupture of the oolemma? Is that an indicator for success? Yes, it can be an indicator for successful injection. No, or immediate rupture, or large funnel formation is associated with higher degeneration and lower fertilization rate.
A difficult injection can sometimes be avoided by laser assisted ICSI. This is where you laser the zona pellucida first and then enter through this opening into the oocyte.
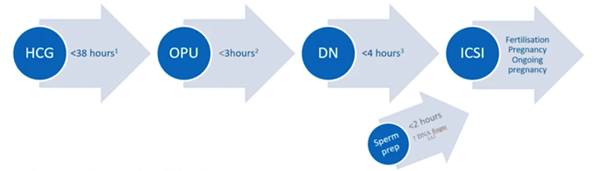
Time to ICSI
Multiple studies have focused on the effect of timing and ICSI outcomes but have had conflicting results. It now seems that fertilization rates, pregnancy rates and ongoing pregnancy rates are better when ICSI is performed less than 38 hours post HCG injection, less than three hours after egg collection and less than four hours after denudation. Sperm preparation should also ideally be performed less than two hours before the actual ICSI.
A recent study by Maggiulli and colleagues showed that there is no influence of procedural timings of operators on the outcomes.
It’s very important that ICSI operators are well trained in the stepwise manner to perform well.
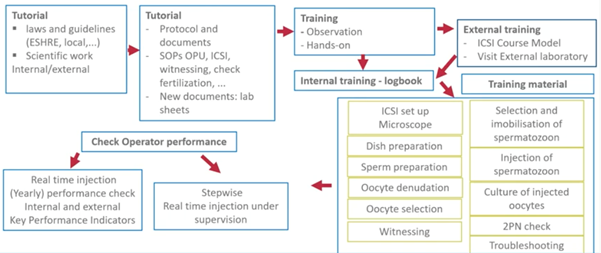
For this, it’s essential that their outcomes are locked, including their timings for each ICSI.
Several indicators to check if we are doing well have been suggested by the Vienna Consensus Committee in 2017. Please understand that these are suggestions and it’s up to each individual laboratory to define their KPIs and reference population that they want to monitor.
- ICSI damage rate
- ICSI normal fertilization rate
- Blastocyst utilization rate
- Implantation rate
- Cumulative pregnancy rate
- Health baby at home rate
When you look at these KPIs, there will be a delay in effect on the outcomes of two to three weeks for the lab KPIs like fertilization and oocyte damage rate. There will be a delay of four to six weeks for the clinical KPIs like pregnancy rate and even longer of course for the cumulative pregnancy rate and healthy baby at home rate.
It is, however, essential that you monitor these qualities with these KPIs to see if the ICSI and the operators in your lab and clinic are performing well.
Training and competency
Continual monitoring of competency is also key. The KPIs I just mentioned can be used for this.
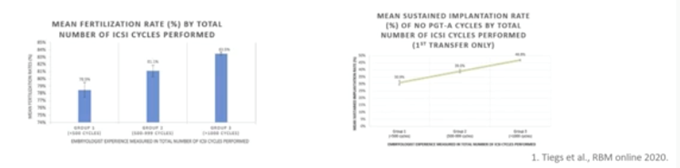
Tiegs and Scott demonstrate here that increased ICSI operator experience is associated with higher fertilization rate, sustained implantation rates and lower likelihood of failed fertilization and usable blastocyst development.
Alternatives and automation
Before I summarize, we should also look at the future of ICSI.
Piezo
The Piezo injection system, where the Piezo effect leads to crystal deformation in response to an externally applied voltage, will propel a microinjection needle tip forward in a precise and very rapid movement. It’s being applied in multiple laboratories around the globe.
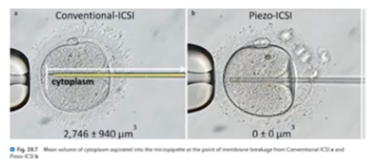
A recent review by Fuji et al showed that with this technique, less oocytes are damaged, embryo utilization rates are better and the health of the children is fine.
There is also a drive towards automation of ICSI. Until now, the following techniques which I describe here are still in the developmental and design and/or experimental phase:
- Microfluidic denudation of oocytes
- Microfluidic single sperm selection
- Robotic sperm immobilization
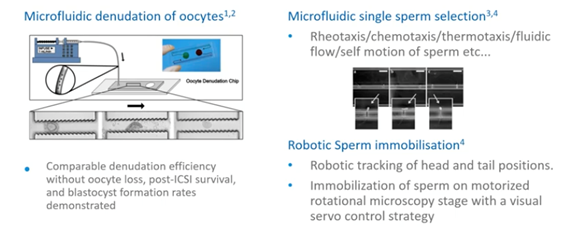
There’s also oocyte trapping with microfluids, real-time electrical resistance measurements of oocytes and the use of artificial intelligence in the development of automated ICSI.
Summary
Now I will summarize the key points.
- ICSI is a high-precision assist reproduction laboratory technique. It requires additional laboratory experience, resources, effort and time. It’s being used more and more globally for non-male indications.
- High quality equipment, media and pipettes are to be used.
- Preparation and selection of spermatozoa and oocytes is important.
- Stress to the gametes should be kept to a minimum.
- All lab conditions should be monitored continuously.
- Witnessing steps are to be implemented to avoid adverse events.
- Training in ICSI and monitoring of operator competence is key to success.
- Automation of ICSI is under development.
About the Presenter: Martine Nijs PhD
Dr. Martine Nijs is an ESHRE accredited Senior Clinical Embryologist.
In the past, she has worked as a Scientific, IVF and Andrology Lab Director in Nij Geertgen, Netherlands and the Genk Institute for Fertility Technology, Belgium. She was also a Senior Embryologist at the Schoysman Infertility management team in Belgium and the co-founder and past director of the Embryolab Academy in Greece.
Martine is a member of the Editorial Board Faculty 1000 Research, an ad hoc board member of 10 international journals and an active member of eight professional societies. She is now a valued member of the CooperSurgical team where she works as our Global Training Manager.
Useful links
If you would like to explore any of the products / references mentioned in this article, please click on the links listed below:
- Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte: G Palermo and colleagues
- Male factor infertility
- ART Media
- Andrology
- Culture Media
- Cumulase
- Multi-well dish
- Pipettes
- Pre-equilibrated liquid oil
- Witnessing of the ID
- Reduced fertilization rate
- High aneuploidy rate
- The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting
- The ICSI procedure from past to future: a systematic review of the more controversial aspects: Patrizia Rubinio and colleagues
- Making ICSI Safer and More Effective: A Review of the Human Oocyte and ICSI Practice: Mara Simopoulou
- IVF Equipment
- Stereomicroscope
- Incubators
- Micro manipulators
- Antivibration table
- Low energy Saturn 5 laser system
- Heated stage warming plates
- Micro manipulation pipettes
- ICSI pipette
- Witnessing system
- SpermSlow
- PVP drops
- HEPES medium
- Origio Handling HSA
- Lifelocal Mineral Oil
- PICSI dish
- Intracytoplasmic sperm injection outcome versus intracytoplasmic morphologically selected sperm injection outcome: a meta-analysis: Amanda Souza Setti and colleagues
- Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction: Cochrane review
- Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction: A later Cochrane review
- Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial: PUBMED UK study
- Hyaluronan-rich medium
- IVF Equipment: Thermosafe
- The effect of ICSI-related procedural timings and operators on the outcome: Roberta Maggiulli and colleagues
- The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators: Vienna Consensus Committee
- Evaluation of fertilization, usable blastocyst development and sustained implantation rates according to intracytoplasmic sperm injection operator experience: Tiegs and Scott
- Evaluation of the effect of piezo-intracytoplasmic sperm injection on the laboratory, clinical, and neonatal outcomes:: Yoshitaka Fujii et al

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
