Introduction
Cryopreservation is integral to providing a safe, efficient, and comprehensive clinical service in assisted reproduction, encouraging elective single embryo transfers, enabling the donation and transport of gametes and embryos, allowing the incorporation of genetic testing and, most importantly, preserving fertility. Cryoprotectants, or cryoprotective agents (CPAs), are organic solutes that modulate the transition between water and ice and, thereby, minimise the damage that cells and tissues might otherwise suffer during cryopreservation (Table 1)¹.
They achieve this primarily by inhibiting intracellular and extracellular ice formation, but they also help preserve cell membranes and promote vitrification. However, further development of successful vitrification protocols is dependent upon a proper understanding of the mechanism of action of CPAs and their biophysical interaction with specific cell types², particularly with respect to the risks of osmotic shock and toxicity during dehydration, cooling, warming and rehydration.
Non-permeating and cell-permeable cryoprotectants
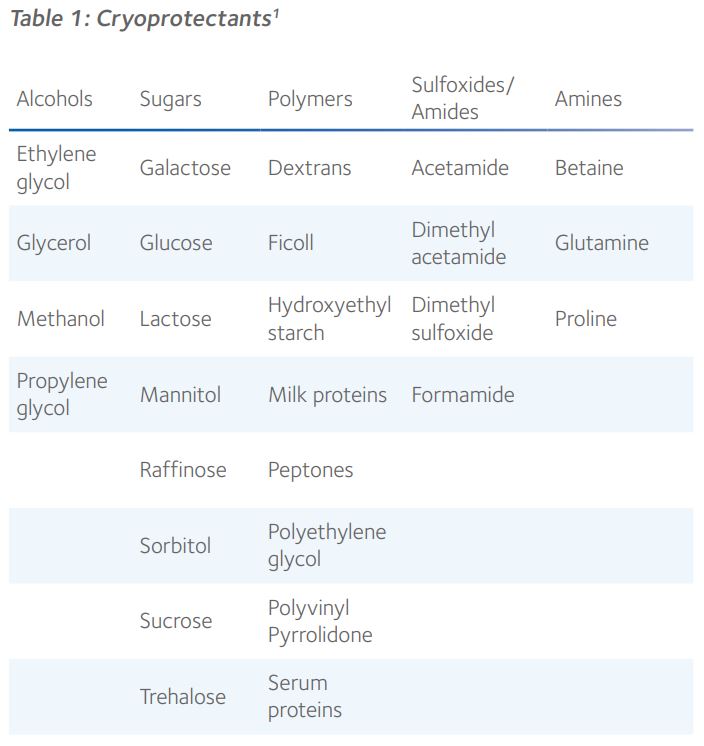
By virtue of their molecular size and ability to diffuse through cellular membranes, CPAs occupy either the intracellular or extracellular space, cell membranes generally only being permeable to smaller molecules such as ethylene glycol, glycerol, dimethyl sulfoxide (DMSO) and 1,2-propanediol, also known as propylene glycol (Figure 1).
Ethylene glycol, propylene glycol and glycerol are kosmotropic, which means they possess anti-freeze properties by forming strong hydrogen bonds with water, thereby competing with the hydrogen bonds that connect water molecules. This effectively disrupts the potential for intracellular ice since specific alignment of water molecules is necessary for crystalline ice formation. DMSO is an aqueous, hydrogen bonding, polar organosulfur compound, with very high permeability through the cell membrane that has been used to reduce ice formation during diverse applications in cryobiology¹. These cell-permeable cryoprotectants all have a molecular mass below 100g.mol-1.
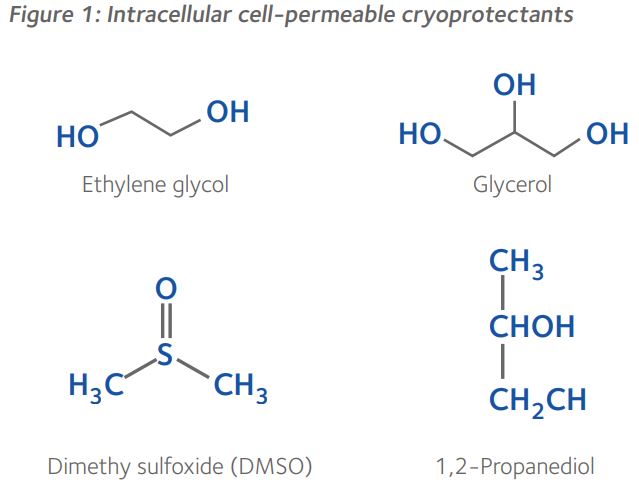
In contrast, the disaccharides sucrose and trehalose, have a molecular mass of 342.3g.mol-1 and 342.296g.mol-1, respectively, and are unable to diffuse across the cell membrane. These non-permeating cryoprotectants influence the osmolality of the extracellular environment, thereby controlling osmosis during dehydration and rehydration. Though sucrose and trehalose have an identical chemical formula, they are composed of glycoside-linked fructose and glucose rings, and two glycosidic linked glucose rings, respectively (Figure 2). Generally, at the same molar concentrations, non-permeating cryoprotectants such as disaccharides and long-chain polymers are less toxic to cells than permeating cryoprotectants and, furthermore, reduce the concentrations of cell-permeable cryoprotectants necessary for vitrification¹.
Molecular mechanisms of interaction between cryoprotectants and cells
During cooling, non-permeating CPAs promote colligative freezing point depression and vitrification by increasing cellular solute concentration via dehydration, and by increasing carrier solution viscosity, respectively, but are also believed to adsorb to the outer cell membrane, thereby protecting the cell from extracellular crystal lattice ice formation1,2. Cell-permeable CPAs have various mechanisms of action: in addition to reducing the temperature at which cells freeze, they raise the temperature at which cells vitrify, and some increase cell membrane permeability, which facilitates cell dehydration2. Furthermore, non-permeating disaccharides help maintain cell membrane integrity during dehydration by lowering membrane lipid phase transition temperatures via non-specific osmotic and volumetric effects1,2. Some cell-permeable CPAs prevent membrane damage by inhibition of adjacent cell membrane fusion via interaction with phospholipids within the lipid bilayer1,2. Cell-permeable CPAs also protect intracellular organelles, such as the stability of the meiotic spindle within oocytes, but their interaction is complicated by the concentration and temperature at which they are deployed1.
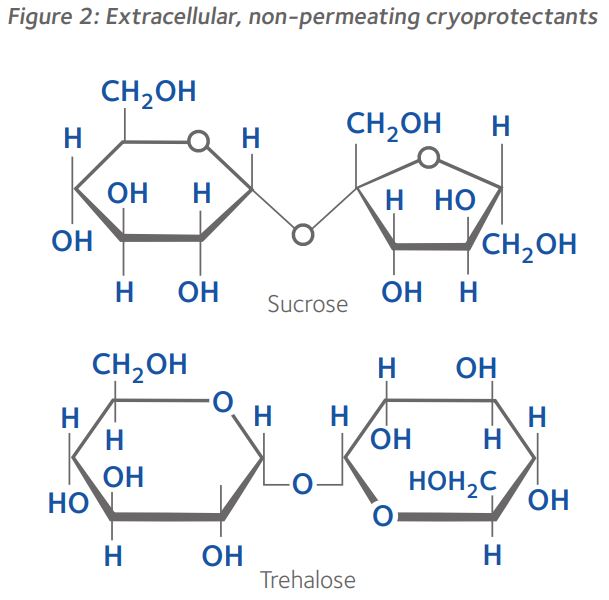
Interestingly, the mechanisms of interaction of different cell-permeable CPAs with cell membranes varies, particularly in their interaction with water within the cell2. Furthermore, depending upon the cell type, CPAs enter the cell by simple diffusion or by facilitated diffusion via channel pathways (Figure 3)3.
Using the mouse model, it has been observed that water and CPAs move slowly across the plasmalemma of oocytes and early cleavage stage embryos principally by simple diffusion through the lipid bilayer3.
In contrast, it was found that glycerol, ethylene glycol and DMSO move rapidly across the plasmalemma, principally by facilitated diffusion via aquaporin 3 in the former two, and via channels other than aquaporin 3 in the latter3. Interestingly, simple diffusion is the only means by which propylene glycol moves across the plasmalemma, albeit at a faster rate during later stages of development3. Furthermore, there appears to be some species differences with respect to the movement of different CPAs across the plasmalemma, though their movement patterns are more stage-specific than species-specific3. Nevertheless, it appears that the general trend is for faster movement of CPAs across the plasmalemma as embryos reach the morula and blastocyst stages of development, which may partly explain why it is usually easier to vitrify blastocysts than oocytes5.
To avoid intracellular ice formation, toxic exposure to CPAs and osmotic shock, the developmental stage-specific permeability of the plasmalemma is crucial to optimisation of vitrification protocols, especially within oocytes which may be uniquely sensitive to transmembrane water fluxes during equilibration and dilution of CPAs. During warming, intracellular cell-permeable CPAs once again protect cells from ice crystal formation.
Figure 3: Movement of water and cryoprotectants across
the plasmalemma of mouse oocytes and morulae3.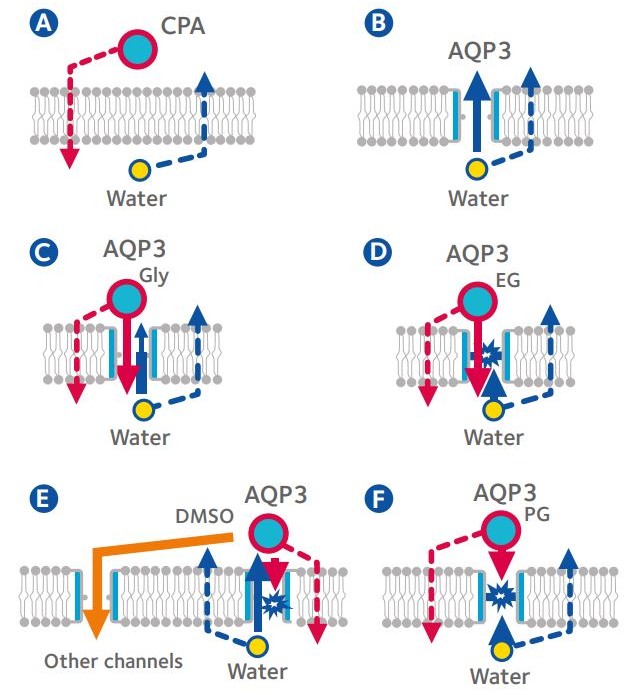
A: Oocyte in solution containing cryoprotective agent (CPA); B: Morula in solution containing sucrose; C: Morula in solution containing glycerol (Gly); D: Morula in solution containing ethylene glycol (EG); E: Morula in solution containing dimethyl sulfoxide (DMSO); F: Morula in solution containing propylene glycol (PG); Dotted lines: simple diffusion; Solid lines: facilitated diffusion via channel pathways; AQP3: Aquaporin 3 (Modified from Kasai & Edashige, 20104)
Clinical application of cryoprotectants
In view of the variation in mechanisms of action of different CPAs and their potential toxicity at higher concentrations, vitrification protocols have been optimised by deploying non-permeating and cell-permeable CPAs in various combinations and at different temperatures, for both cooling and warming. Indeed, mixtures of CPAs have been demonstrated to confer greater protection to cell membranes with reduced toxicity, especially with stepwise addition and removal, and at lower temperatures<sup>1</sup>.
Consequently, there is general consistency in composition of vitrification kits between different manufacturers (Table 2).
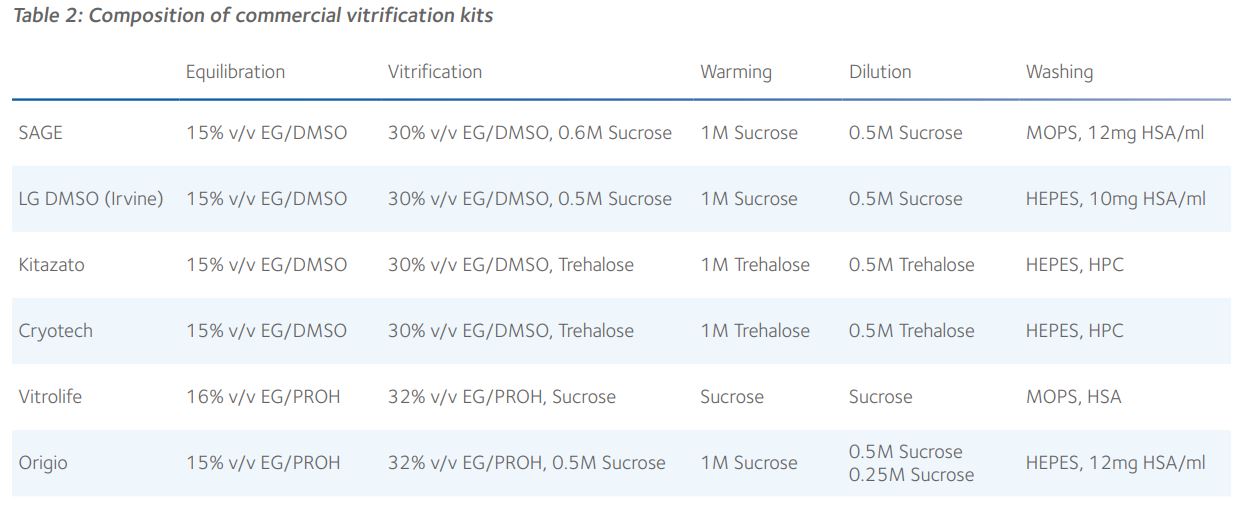
It is evident that the key CPAs in different vitrification kits are very similar and that their concentrations are almost identical and, therefore, the rates of dehydration and rehydration during cooling and warming must be very similar. Given that the molecular masses of sucrose and trehalose are virtually identical, their equimolar impact upon the osmolality of the external environment will be the same. Indeed, there is no clear published evidence in favour of any combination of CPAs and it is perfectly feasible to optimise the use of any vitrification kit in any embryology laboratory. Furthermore, there is now good evidence that vitrification warming kits are interchangeable, which suggests that a universal warming protocol can be applied, regardless of the cooling protocol used for vitrification of blastocysts and oocytes<sup>6,7</sup>.
Summary
Provision of a clinical service in assisted reproduction is very much dependent upon a competent program of cryopreservation. In that respect, proper understanding of cryoprotectant-cell interaction and optimal deployment of cryopreservation kits containing mixtures of non-permeating and cell-permeable CPAs is critical to cryo-survival and viability of gametes, embryos, and other reproductive tissues.
Indeed, vitrification has markedly improved cumulative live birth rates in assisted reproduction over the past 10 years.
References
- Elliott, GD et al. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017; 76: 74-91.
- Raju, R et al. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim Biophys Acta Gen Subj. 2021; 1865: 129749.
- Edashige, K. The movement of water and cryoprotectants across the plasma membrane of mammalian oocytes and embryos and its relevance to vitrification. J Reprod Dev. 2016; 62: 317-21.
- Kasai, M. & Edashige, K. Movement of water and cryoprotectants in mouse oocytes and embryos at different stages: relevance to cryopreservation. In: Chian R-C, Quinn P (eds.), Fertility Cryopreservation. Cambridge: Cambridge University Press. 2010; pp. 16-23.
- Rienzi, L et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017; 23: 139-55.
- Canosa, S et al. Are commercial warming kits interchangeable for vitrified human blastocysts? Further evidence for the adoption of a Universal Warming protocol. J Assist Reprod Genet. 2022; 39: 67-73.
- Parmegiani, L et al. “Universal Warming” protocol for vitrified oocytes to streamline cell exchange for transnational donation programs: a multi-center study. J Assist Reprod Genet. 2020; 37: 1379-85.
 Steven D Fleming PhD
Steven D Fleming PhD
Director of Embryology
CooperSurgical Fertility Solutions
Previously, he was the founding Scientific Director of Assisted Conception Australia, and a Senior Fellow at the University of Queensland. After completing his doctorate in 1987, he undertook postdoctoral research at the Royal North Shore Hospital in Sydney. From 1993-1997 he was appointed Lecturer in Obstetrics and Gynaecology at the University of Nottingham in England, where he established the world’s first Master’s degree in Assisted Reproduction Technology with Simon Fishel PhD. From 1998-2008 Steven was the Scientific Director of Westmead Fertility Centre in Sydney and appointed Senior Lecturer in Obstetrics and Gynecology, and subsequently an Honorary Associate in the School of Medical Sciences at the University of Sydney.
He is the recipient of numerous research grants, and an author and editor of several books, book chapters and peer reviewed journal articles. Steven’s research interests include cryopreservation, endometrial physiology, endometriosis and oocyte maturation, among others.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada