Revisiting pH in ART
In this webinar, we revisit the popular topic of pH in ART and we have a presentation by Dr Steve Levett, Director of Clinical Application for CooperSurgical.
This webinar follows on from a previous one that we did earlier in the summer which looked at optimizing culture conditions in the IVF laboratory. That webinar covered pH in some detail and today we’re going to revisit pH, its role in IVF laboratory practice and in maintaining a stable pH to optimize culture conditions. We’ll discuss some fundamentals including:
- What is pH?
- pH and cell metabolism
- How to measure pH in the IVF setting
- pH in ART (Assisted Reproductive Technology)
We’ll finish with a summary at the end and there will also be a bibliography which you can refer to at a later date.
We’ll start at the basic level and look at exactly what pH is.
What is pH?
pH is an acronym which stands for either potential of hydrogen or power of hydrogen, depending on which you prefer. It’s a measure of acidity or alkalinity expressed by the hydrogen ion concentration.
pH is an inverse logarithmic scale and it ordinarily ranges between 1 and 10 to the minus 14. This scale was devised in the 1920s by a Danish biochemist.
Acids are defined as substances that increase hydrogen ion concentration, while bases or alkaline are substances that decrease this concentration.
The pH scale that we use today ranges from pH1 to pH14. Substances with a pH of less than 7 are considered acidic and include things like battery acid, stomach acid and lemon.
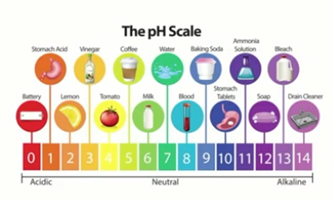
A very simple example is when we taste lemon, we have a very sour taste in our mouths. The reason for that is that about five to six percent of a lemon’s constituent is citric acid. Citric acid has a low pH of around 2.2, which our palette can detect.
Substances with a pH greater than seven are classified as basic or alkaline. This could be ammonia solution or bleach for example.
A pH of seven is considered neutral.
Now we’re going to move on to the role of pH in cell metabolism; something which is, of course, very relevant to the ART laboratory.
pH and cell metabolism
Intracellular (pH¡) and extracellular (pHe) pH
Intracellular pH is, in human embryos, typically around 7.1. The literature varies between 6.95 to 7.1. It regulates protein confirmation, glycolysis and critical metabolic and transport processes in the human oocyte and embryo.
In most commercially available culture media, extracellular pH is recommended somewhere between pH7.2 and 7.4. We use this for human embryo culture.
pH and IVF culture systems
In human embryos, the internal pH of the cells initially follows the external pHe in the culture media. Because of this, fluctuations in the external pH could be seen to affect the embryo’s development.
Embryos do possess mechanisms to regulate their internal pH. This mechanism is an energy-requiring process however which does of course have an impact on the cellular resources within the embryo.
It has been demonstrated in hamster embryos that slight changes in pH for a few hours resulted in relocalization of mitochondria and actin cytoskeletal elements. It has been shown that later stages in embryo development can regulate their pH internally more efficiently than those earlier stages. It’s likely that this is because of the cells having tighter cell junctions.
pH and Buffer Systems
A buffer dampens changes in hydrogen ion concentration. It’s generally a weak acid and it has a conjugate base which can readily absorb excess hydrogen or hydroxide ions. Because of this, a buffer has the ability to maintain pH as well as bring it back to its optimal value through the additional removal of these hydrogen ions. In biological systems, a buffer is absolutely crucial to maintain, in many instances, life itself.
Intracellular (pH¡) and extracellular (pHe) pH
Intracellular and extracellular pH are determined by the media that the embryos are cultured in. In the IVF laboratory, we generally use a bicarbonate-buffered medium which relies on changing levels of CO2 to maintain the optimal pH.
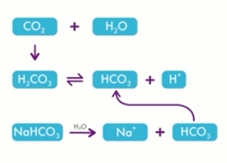
As I just mentioned, most culture media use a bicarbonate CO2 buffering system to maintain target pH. Unfortunately, throughout the time that embryos have been cultured, for most culture media, the ideal pH has not been determined. This does, of course, need to be determined locally by setting the pH to give optimal results.
pH is set by the relationship between CO2 and bicarbonate, and the bicarbonate concentration in the media is determined by the manufacturer. Unless we manipulate the media in some way to change that concentration, the only thing we can do to manipulate the pH in our media, is change the CO2 concentration. Generally, that is what we do and a stable CO2 with a stable bicarbonate concentration will lead to a stable pH in the culture media.
Any changes in either of those parameters will obviously lead to a change in pH which we need to avoid as far as possible.
pH and buffer systems
There are a number of buffer systems other than bicarbonate and CO2. Generally, these are known as zwitterionic buffers. They have the ability to maintain a range of pHs, which is variable by temperature.
As we know in culture media, external pH rises quickly outside of the incubator if we don’t use a zwitterionic or a different type of buffer. If it’s a bicarbonate CO2-based buffer system, then as soon as we take the dishes out the incubator, we lose CO2 and the pH will change rapidly.
Handling media contains a buffer suitable to maintain the external pH within a desired range. pH maintenance using these buffers is temperature dependent. The pKa which is a reflection of the pH, indicates the middle of the buffering range when buffering capacity is at its best.
If you look at the table below, you can see a range of different buffers, the range they cover and the pKa or pH at different temperatures.
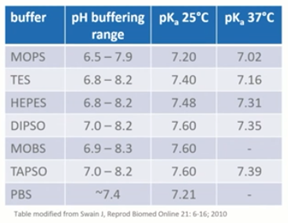
If we take PBS for example, it’s a phosphate buffered saline which has been used for a long time in culture systems. Not just for human embryos, but for many other cell culture systems as well. It has good buffering capacity and has been used extensively in cryopreservation, particularly for slow freezing.
It’s very basic however and lacks many of the other essential components that we now recognize are needed for healthy embryo development. Because of that, long-term exposure may compromise embryo development itself. PBS is something that has really gone out of fashion and we don’t we don’t use it in our culture systems.
On the other hand, HEPES is very commonly used. We use it for any system where we’re manipulating embryos outside of the CO2-rich environment within the incubators. It supposedly has toxicity to human embryos but this is generally linked to light exposure and the presence of riboflavin, which is not relevant for IVF.
The main reason that embryos don’t thrive in long-term culture in HEPES is actually because of a lack of bicarbonate. We know from many years of experience that if it’s used correctly, and as you can see from the table, at the correct temperature, it supports oocyte maturation, fertilization and embryo development.
In this example, HEPES at 25 degrees is outside the range that we would want it to be for good embryo development. 37 degrees is right in the middle of the recommended range.
If we move on to another zwitterionic buffer, MOPS, you can see the pKa is lower than ideal at 37 degrees. If we look, it’s 7.02 which is very close, if not below the internal pH of the embryos. It is suitable to be used at 25 degrees centigrade. It has been shown to affect some cell functions e.g. taurine uptake. It has been shown to interact with DNA but regardless, works very well as a buffer in some systems and some commercially available media.
How to measure pH
One of the questions I’m repeatedly asked when in the field visiting embryology labs, is what is the best way to measure pH in the IVF or embryology lab setting?
How to measure pH and how to select your pH meter
How do we select the best pH meter to give us the most relevant readings in the embryology laboratory?
Some criteria include:
- Accuracy
- We need to know how to calibrate the particular reader we’re using. Is it manual or automatic and is it to be determined at room temperature or at 37 degrees?
- Temperature accuracy within the probe or the apparatus we’re using
- Operation time on the battery
- Whether it will log the data
- Whether you can export that data once the measurements have been done
- Is it suitable to use in our laboratories in terms of its size and weight?
- The cost of doing the pH measurements
- How long it takes to do each measurement
- Shelf life of the buffers or cards that we’re using
- If we’re buying it from a commercial supplier, we always need to take into account the after-sale service and support that we get for that particular piece of equipment to make sure it operates optimally all the time
How to measure pH
There are a number ways of measuring pH, not just with pH meters or as we’ll talk about, blood gas analysers.
I’m sure all of us have had some very basic indicators to work with, one being phenol red, which is generally present in many culture media.
This is a gross indicator of pH. When it comes to determining an exact colour to give an indication of the pH, it’s subjective between operators which means its relatively low accuracy. It also only works with larger volumes because it’s very difficult to see these colours in small volumes.
When culture media was first made in laboratories, before it became commercially available, phenol red was used as an indicator. This meant that the eye of the embryologist was extremely important in determining whether the pH was in the required range between 7.2 and 7.4.
Theirs is, of course, also pH paper. When using pH paper, you have to remove the medium from the incubator, which is going to give you some immediate change in a bicarbonate CO2 situation. This method also works better in large volumes and there’s no calibration of the paper itself. This makes it hard to determine if you’re getting an accurate reading.
Potentiometric pH meters with a reference electrode, are a more sophisticated method of measuring pH. Once again, we have to remove the medium from the incubator which is going to lead to changes if we’re not careful. This is because of loss of CO2.
This method also requires large volumes and manual calibration is needed before use. It generally offers low accuracy of the readings.
We can use pH meters that are designed specifically for Assisted Reproductive Technologies (ART) and culture systems. We can measure in the petri dish or in the incubator which tends to be more accurate. They can mimic some culture systems and styles, but not all and of course, you can get measurements in real time.
More developments are coming in this arena where systems within incubators will give you real-time readings of pH. This helps to give confidence that things are stable within the incubator and within the culture system.
It’s now becoming more common to measure pH with a blood gas analyzer in IVF laboratories. This has some advantages over the traditional pH meters. For example, you only need small volumes. This is an epoch reader, which only requires 93 microliters to give a pH reading.

Generally, you need to take a little more than that to ensure you get a good sample, but it’s still a relatively small volume compared to the others.
Another benefit is that you get a fast reading. Once the sample is on the card that goes into the machine, it takes just 30-35 seconds. It has automated calibration, which is on the cards that the reader uses, it gives very accurate, reproducible results and it logs the data within the reader itself. Printers are available and you can download the information and store it for later.
pH in ART
Once we’ve established that we can measure pH accurately, we need to look specifically at pH in the IVF laboratory and in the IVF culture system.
We’ll look at IVF culture systems in relation to egg collection, in andrology, micromanipulation, embryo transfer and cryopreservation.
IVF culture systems
First of all, we’ll talk about pH in the culture system. This is the area that our embryos are going to sit for the longest period of time. It’s also the area that we need to pay particular attention into maintaining a stable and narrow range of pH throughout the embryo culture time frame.
The below image gives you an idea of the number of factors which can affect the outcome of IVF in the IVF laboratory. This was put together by Don Rieger in 2012 and it gives an indication of the number of factors in each procedure that can impact the overall outcome of the IVF clinic and the success of the treatment for our patients.
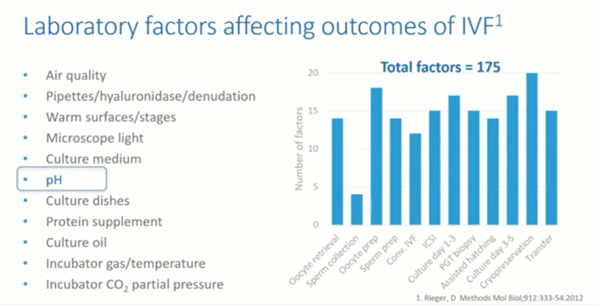
As you can see, it covers many areas including air quality, culture mediums and of course, pH. We also have plasticware, oil and other parameters that we use. It gives an indication that there are a significant number of factors that we need to monitor within the IVF laboratory to ensure that we get the best outcome for our patients.
Factors affecting pH in culture media
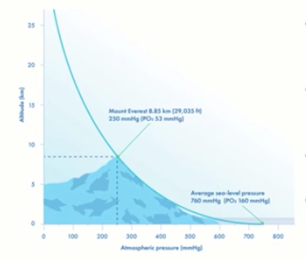
Altitude
Altitude plays a major part in determining the concentration of CO2 that we need to give us the optimal pH in our culture system. As the altitude increases, the partial pressure of the gas decreases.
When people climb Mount Everest for example, most of them must use oxygen to get to the top. This is because the partial pressure of oxygen at this extreme altitude is very low, which makes breathing very difficult.
The same concept applies in the embryology lab and in our culture systems. As altitude increases, we need to increase CO2 concentration to maintain the pH that we’ve determined is optimal for that culture media.
One of the questions we’re always asked is ‘what CO2 concentration should I use in my culture system?’ As we’ll see, altitude isn’t the only issue. There are many other factors, and this is something that needs to be determined locally, it isn’t something that can be determined from afar.
Equilibration
One of the other major factors in our culture system is equilibration. Most IVF clinics use microdrop culture under oil. This has many benefits for the actual culturing of the embryos and long-term culture, particularly now as we’re aiming to potentially culture embryos from day zero, or day one up to day five, six or seven. Because of this, we need to use a very robust system.
Using small drops and large volumes of mineral oil to protect the system has an implication on the equilibration time of our dishes. The equilibration falls into three sections; equilibration, set point and stabilization. This generally takes much longer than many people realize and there has been a lot of work done to determine optimal equilibration time, such as the paper published by Jason Swain in 2012.
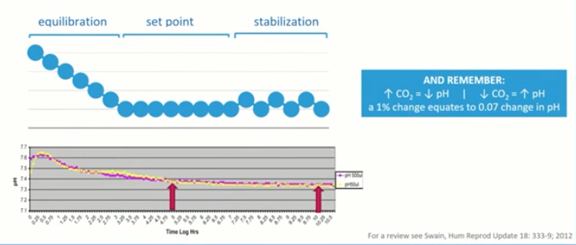
We’re looking at many hours, certainly not minutes, to be certain of reaching equilibrium of your culture dishes. We need to be in the region of somewhere between 14 and 18 hours to guarantee that the dishes have reached equilibrium.
Stabilization is of course going to be a factor when we open and close the incubators, particularly if we use the big box type incubator. We need to ensure that stabilization is reached and that if we increase CO2, we decrease the pH and vice versa.
Generally, a one percent change in CO2 concentration equates to about a .07 change in pH at sea level.
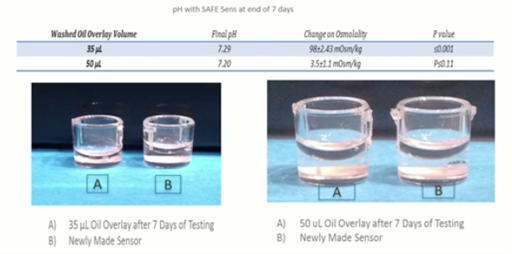
Volumes
The take home message here is that when you’re using low volumes of media, you need to ensure that you have a good volume of oil overlay to protect those. The smaller the droplet that you use, the greater the surface area to volume ratio, and the greater the risk of evaporation and therefore changes in both pH and osmolality over time.
Other factors affecting pH in culture media
Other factors which can affect the pH in culture media include:
- Protein supplementation. If we supplement the media ourselves in the laboratory, we dilute the bicarbonate in the culture media and that will therefore have an impact on the pH settings and the CO2 concentration that you need
- The frequency of incubator door openings has been shown to have a significant impact
- Loss of CO2 will cause an increase in pH
- How you measure the CO2 is of critical importance
- Where the air conditioning units are placed in the lab
- Where you make the dishes can have an impact if you get evaporation as you’re making the small droplets
- Temperature changes within incubators can have an impact on pH. We are of course moving more to benchtop incubators which have greater control than the box type incubator
- Whether we have any contamination present in our culture system which is fortunately something we don’t see so much nowadays. This was a constant problem in the early days of embryo culture
- Whether we use humidified or non-humidified incubators is going to be a relevant issue
- How we measure the pH is going to impact the overall stability and optimization of our culture system
pH and oocyte collection
Now we’re going to move on to pH and egg collection.
The Intact Oocyte Cumulus Complex (OCC) is known to be less vulnerable to changes in pH than denuded oocytes. Embryologists who work in the field will know the control of temperature during a collection is a much more pressing concern than control of pH. This was more of an issue in the early days when we did egg collection via laparoscopy. Nowadays, with the transvaginal ultrasound and the transvaginal egg collection, this is a problem that has diminished.
Flushing media that we use is usually buffered and we should try to avoid saline whenever possible. Hartmann’s or Ringer’s solution is not buffered and therefore is open to changes in pH.
After we’ve identified the egg in the cumulus complex, they’re washed and kept in our zwitterionic buffer – either HEPES or MOPS. Once they’ve been washed through culture media, they’re put into the bicarbonate buffered culture media to await fertilization through IVF or ICSI.
If you’re going to do this in the flow hood, then you need a supply of gas to blow over the media to maintain the correct pH.
Sperm preparation
If we move on to sperm preparation, things are a little different here.
Media that’s used in the preparation and handling of sperm needs to maintain the health and vitality of the sperm and cause as little damage as possible.
Generally, we use gradients to separate out the healthy, normal sperm from the rest of the debris and immotile sperm. Alternatively, we can use a swim up technique. The swim up and sperm washing media needs to provide energy, support hyperactivation and capacitation of the sperm, and safeguard against any damage due to antioxidants. This is a somewhat different environment to those that we see for the vitality of embryos.
Sperm prefer a very different environment and conditions from those of eggs. If we look at the table here, looking at hyperactivation, you can see that the sperm show much better hyperactivation in a pH that’s higher than we would want for embryo development, embryo culture and even for the handling of oocytes.
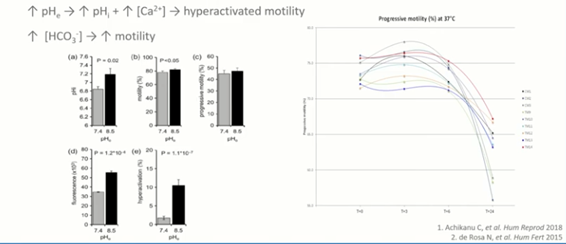
A pH of 8.5 is generally better and this is more reflective of what the sperm will see in vivo. If we want to get the best possible sperm for our treatment, we need to take these different physiological characteristics into account.
For the handling of sperm, the media should support all of the functions we’ve mentioned and therefore needs to potentially contain albumin and bicarbonate and have an alkaline pH (above eight).
Media optimized for sperm preparation
As you can see in the table, pH optimized for sperm preparation is a new sperm handling media produced by CooperSurgical.
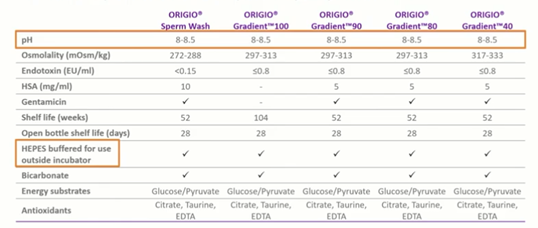
One of the standout features we can see is that the pH here is much higher than we would recommend for embryo development. We can see the pH ranges from 8 to 8.5 which is perfect for healthy sperm. We also have HEPES within the media for use outside of the incubator. This is going to be used in an environment devoid of CO2. Just in the normal background CO2 levels, bicarbonate is present – but this is for the health and development of the sperm, not for a buffering capacity.
Micromanipulation
Denuded mature eggs lack the ability to regulate their internal pH. This means that they’re dependent on the external pH in the culture media. Because of this, it’s very important that once we’ve denuded eggs, to avoid any changes in pH wherever possible. We also need to be careful when we’re moving and handling the denuded eggs to maintain the pH in the various dishes that we use.
pH is known to affect cytoskeletal elements in the oocyte. These are of course involved in positioning the meiotic spindle. This means that procedures like denudation and ICSI, where we’ve removed the protective cumulus cells, can create an environment where we could see chromosomal abnormalities formed if we don’t maintain a stable and optimal pH during the whole procedure.
It is possible to use bicarbonate buffered media for doing ICSI but this does limit the time that you can have the dishes outside a CO2 controlled incubator. It’s much more common to use a zwitterionic buffer – either HEPES or MOPS – because this protects the pH by temperature and not by CO2.
Using smaller drops can be problematic because of evaporation, which can cause potential changes.
We also want to maintain the temperature as closely as possible to maintain a stable pH for the whole procedure. To do this, overlay with 6 ml of pre-warmed mineral oil. This should be a ratio of one oocyte or embryo per drop to limit the exposure of the aperture for light.
Then we need to equilibrate the dishes to the required temperature (37°C) for the manipulation prior to any usage of that dish. Generally, we need at least 30 minutes to reach equilibrium, assuming that the media and the oil have been pre-equilibrated. This is to ensure that you get a stable environment for the whole time you’re manipulating the gametes or the embryos here either in ICSI or with embryo biopsy.
When we move eggs or embryos after the washing to remove the buffer, put them into number drops. Post micromanipulation, we need to wash through several drops of our bicarbonate buffered culture media to remove any excess buffer.
As with everything, when we move gametes or embryos from one place to another, we need to have a robust witnessing system in place. We also need to work as quickly as we possibly can without risking any adverse incidents to reduce the time that everything is outside of the CO2 incubator. Again, this is to minimize any changes in pH.
pH and embryo transfer
Although, at later stages of development, embryos are less vulnerable, we should still take great care to maintain the pH, temperature and osmolality as much as possible in this latter stage before we put the embryos into the uterine cavity.
Most laboratories would just load the embryo transfer catheter in their standard bicarbonate buffered culture media. This would enable them to work quickly, ensuring that the embryos are transferred from the dish into the uterine cavity as quickly as possible.
It is, of course, possible to use a HEPES buffered media where temperature needs to be controlled very carefully to maintain the pH. But one area where care is significantly needed however, is during frozen embryo transfer. This is because cryopreserved and warmed or thawed embryos do have a reduced ability to regulate their internal pH for about three to four hours after they’ve been thawed or warmed. During this time, we need to be very careful to maintain a stable pH so we don’t cause any undue damage to those embryos prior to transfer.
pH and Cryopreservation
A phosphate buffered solution was commonly used in early slow freezing systems. Now, most commercial kits use a zwitterionic buffer (either MOPS or HEPES) to control pH during cryopreservation (including sperm cryopreservation).
Cryopreserved and thawed embryos have a reduced ability to regulate their internal pH for about three to four hours after they’ve been thawed or warmed. During this period, we need to ensure that we maintain a stable culture environment with no changes in pH or osmolality. We also need to try to control temperature as much as possible.
Summary
To summarize:
- Critical metabolic and transport process within the human oocyte and embryo are regulated by pH
- We’ve shown that denuded eggs are unable to regulate their internal pH
- Cleavage stage embryos can’t regulate internal pH as well as the latter stages or the blastocyst stage
- Organic and inorganic buffers can maintain a stable pH in solution
- We use either HEPES or MOPS in this scenario. They’re not reliant on CO2, but they are reliant on temperature to maintain the correct pH. This means that we still need to take great care if we’re using these particular types of buffers
- If you’re measuring pH in your laboratory, a blood gas analyzer seems to be the most efficient way to do this. The exception is if you have an inbuilt system within your incubator which gives accurate, reliable, and reproducible results in a scenario that very closely mimics the culture conditions that the embryo would see
- We’ve also discussed that there are many laboratory variables which can impact the pH within the culture media
- Temperature and osmolality will play a part and can be detrimental if not monitored and maintained in a stable manner
About Steve Levett
Steve Levett has a keen interest in lab optimization, QC procedures, optimization of results and supporting embryologists. His research interest has been in fertility preservation since working with Professor Roger Gosden in Leeds. As mentioned earlier, Steve is now the Director of Clinical Applications at CooperSurgical Fertility and Genomic Solutions.
After completing his PhD in Molecular Immunology in 1989, Steve obtained a position as Postdoctoral Fellow at York University. He then took up his first embryology role in the early 1990s at St James’s University Hospital in Leeds. He became Lab Manager soon after and then went on to manage other embryology labs in the public and private sectors, both in the UK and in Canada
From 2006 to 2014, Steve worked for Wallace (then part of Smiths Medical), becoming Global Business Director in 2010. He joined Ferring in 2014 as UK National Business Manager for Obstetrics and Fertility. 18 months later, he joined Life Global as Vice President for International Sales.
Find out more about Steve Levett.
Find out more
The following are mentioned throughout the text above and/or are particularly relevant to it. To find out more about each item, please click the link.
- Reference
- ‘Optimizing your Embryo Culture System’ webinar
- Whitepaper: ‘pH in Medically Assisted Reproduction Procedures: Its Importance, Measurement and Control’ by Jason E. Swain, PhD, HCLD and David Morroll, PhD
- Products
- Procedures

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada

