In this webinar, Steve Levett, PhD provides an introduction to factors affecting embryo culture, including pH and osmolality, and how these can be optimized in the clinical IVF laboratory.
Webinar Transcript: Optimizing your embryo culture system
This webinar explores how you can optimise your embryo culture system. It also introduces the factors that can limit the success of embryo culture and explore how these can be controlled.
The laboratory procedures for setup of cultures including dish preparation, control of pH and influence of oil overlays will be considered. In present circumstances we put a lot of pressure on our culture system to perform. We mainly go to day five, day six or even day seven culture to give us blastocysts that will give better choice of embryos for embryo transfer, reduce multiple pregnancies and to allow material for genetic testing. Hopefully, then, this leads to giving our patients the best possible chance of success and delivering a healthy, normal baby.
So the presentation will take the form of these three sections
- Media and consumables
- Media pH and CO2 concentration
- Oil and osmolality
Obviously, with the nature of this topic it’s only possible to touch on some of these points and, with the time constraints, we’ll cover some areas that will hopefully give food for thought for those returning to the laboratories after the unprecedented [coronavirus] situation as we have today.
Optimised Embryo Culture Media and Consumables
What is embryo culture media as a starting point and how do we optimize its use within the culture system that we have in our laboratories?
- Embryo culture media is a component of in vitro fertilization (IVF)
- Embryos are cultured in this artificial environment which consists of substrates including glucose, pyruvate, lactate etc.
- The addition of other components such as amino acids, nucleotides, vitamins etc to hopefully enhance the performance and improve embryonic growth and development
- Also added things like antioxidants, antibiotics, macromolecules, proteins etc can be added
- Optimization of the culture media is essential by optimizing all the other components of your culture system, including the environment and equipment used.
We have to bear in mind that every commercially available media has these ingredients in different concentrations and different factors that are added.
We have to remember that culture media is an artificial environment for embryos to grow and it has to try to mimic what happens in the dynamic and complex environment in the in vivo situation. Fortunately for us, the media that’s currently available allows us to support those things such as sperm function, egg mediated cleavage stages, genomic activation and the early differentiation of cell types. And, of course, it provides a minimum requirement for competency in these different cells.
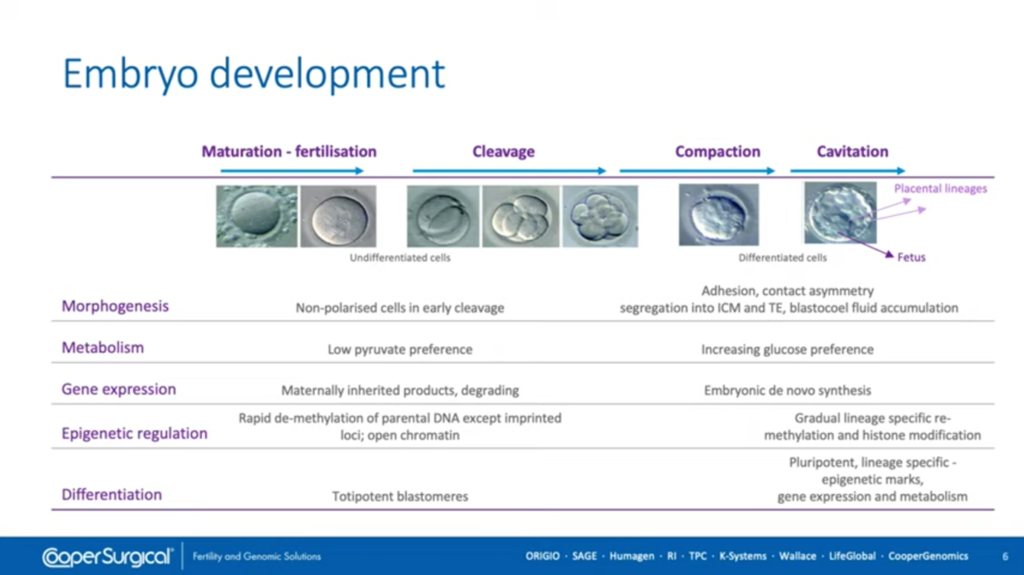
This slide shows you, in detail, some of the embryo development that has to be supported. We go from maturation of eggs which has the cumulus cells attached, to fertilization (so the media has to support sperm function as well if we’re doing IVF) and then, of course, the early cleavage stages, the activation of the embryonic genome, through to compaction and then cavitation and hopefully the formation of high-quality blastocysts which will allow us to transfer embryos with high development potential / high implantation potential and give us the material also to do genetic testing should we want to do that in our laboratory.
Embryo culture media is not the be-all-and-end-all (although a major part of what we do). We have to take into account all of the other laboratory factors that can affect IVF outcome.
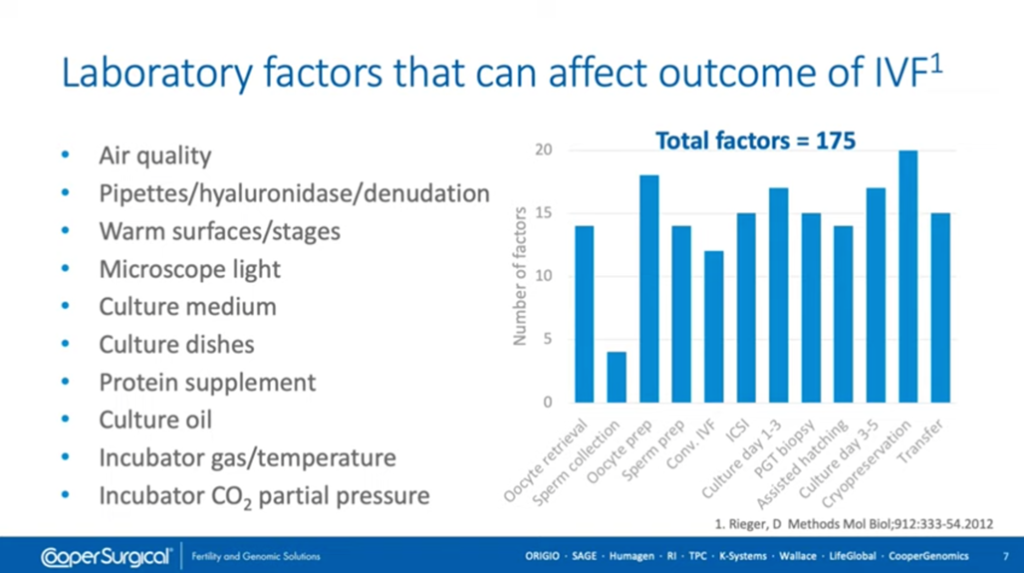
This slide was put together by Don Rieger, a former colleague of mine, and it gives some indication of the complexity of the situation that we have. We have to take into account the environment (including air quality) and all of the equipment that we use. As you can see, culture media is only one of those factors that can affect the outcome. All of these need to be optimized and all of them need to be monitored and controlled regularly to ensure that we keep the optimal situation once we’ve established it in our laboratory.
To reiterate, embryo culture is more than just the media. It does involve all of the other factors; the consumables, the equipment, the atmosphere and everything else. We should bear in mind that we need to minimize insults and stresses to the gametes and embryos to give the best possible outcome for our patients.
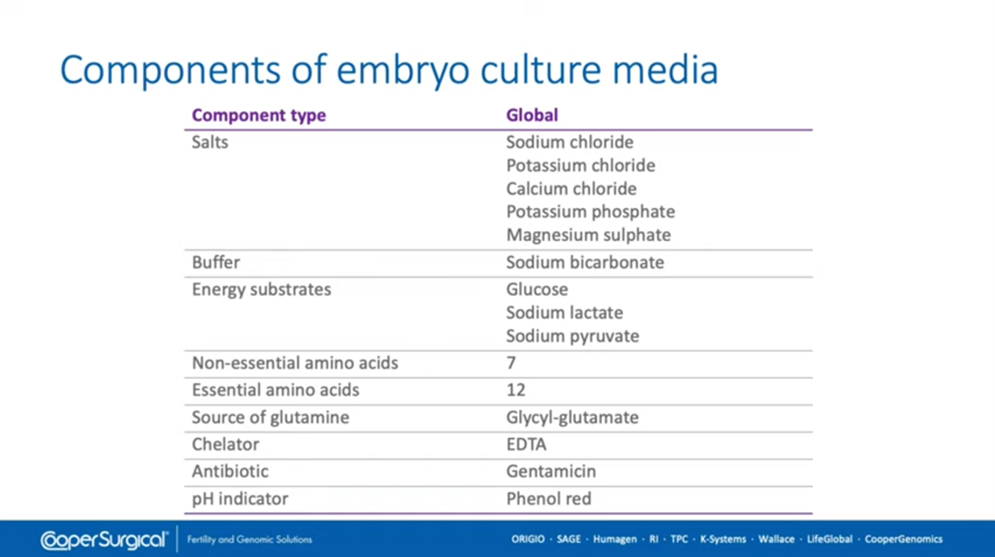
Embryo media is made up of some very basic components including salts as you can see here. Generally, for the culture of embryos through to day five, day six, or even day seven after fertilization the media contains sodium bicarbonate as a buffer. It will have energy substrates available and, of course, nowadays essential and non-essential amino acids. Glutamine is usually in a dipeptide form to avoid the formation of ammonia over extended culture. There will also be a chelator, some antibiotic and, of course, a pH indicator which most media has, but not all.
And, of course, these are the basic components of embryo culture media. They’re not the only ingredients and the concentrations etc that I mentioned earlier are different in in every formulation that’s commercially available.
Comparison of Single Step Media and Sequential Embryo Culture Media
Single step media is where a single media is used throughout the embryo’s development, and sequential media is where the media is in two forms; generally a cleavage media and a blastocyst media.
Our single step media can be used in two ways; either where we renew the media on day three or we use it continuously after fertilization through to when the embryo’s ready for embryo transfer for genetic testing or for vitrification.
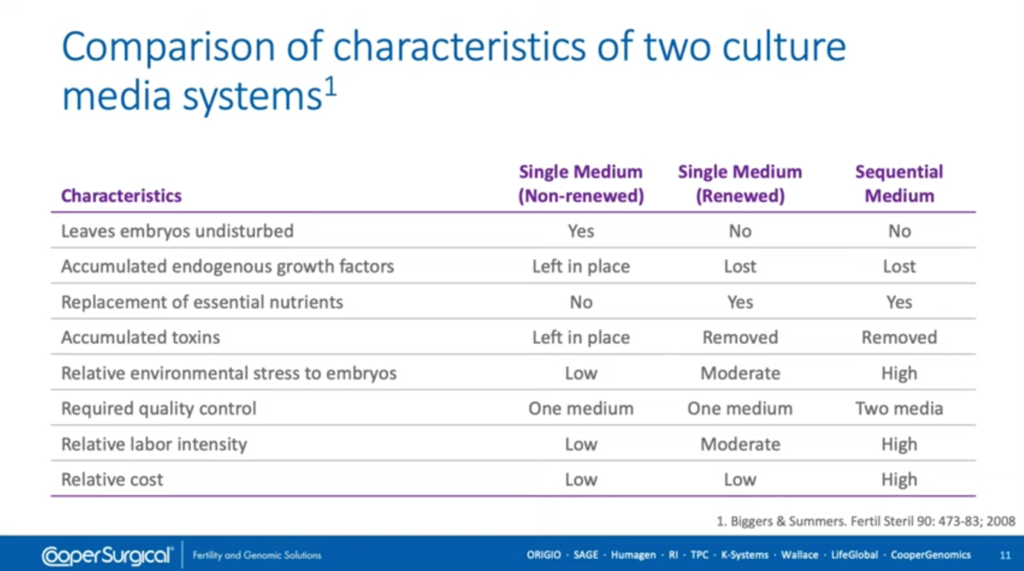
And this table just gives a comparison of the characteristics of these different systems. So, as you can see here, if we use single media undisturbed, we have the potential to accumulate any endogenous growth factors that might be present in the media throughout the embryos development, but we don’t replace any of the nutrients which we do if we renew single step media or use sequential media.
If there are any accumulated toxins, of course, they’re left in place when we don’t renew the media and they’re removed on day three when we do. And again, you can see the differences in the different types of media in terms of what we need to do in quality control, labour intensity and the relative costs. These come from a paper published in 2008 by Biggers & Summers.
Of course, we also have to consider what we culture our embryos in. Not all plasticware is the same so we should wherever possible use IVF-grade plastic ware. We should consider the materials that these dishes are made from (with low VOC-emitting plastic ware) and ensure that the quality control of that dishware is of the highest quality. We have LAL testing looking at endotoxins and we have a Mouse Embryo Assay to ensure that when our embryos are cultured in in the dishes that they have the best chance of developing normally without any insults from the environment itself.
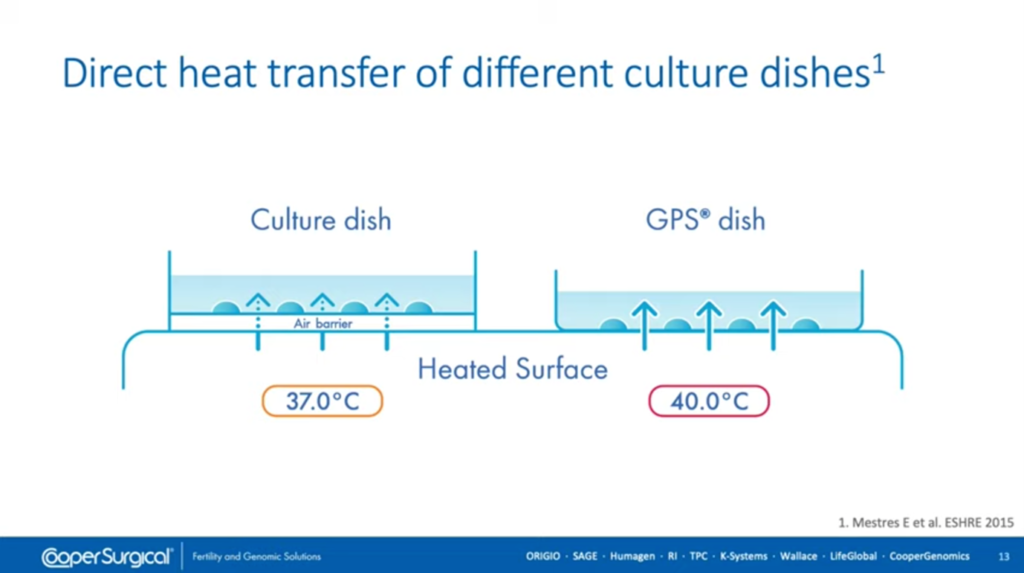
Do dishes make a difference? Are all dishes the same? Well, there are some publications – this one [above] that was presented at ESHRE in 2015 – looking at heat transfer between dishes and, of course, the design of the dish can have an impact on how they perform.
Those that are not designed specifically with intact wells integrated within the dish itself have a different setup. You can see from the illustration here, the dish on the left has an air barrier underneath which does provide some insulation from heat, whereas the dishes with pre-formed wells generally have direct transfer of heat. This can make a difference in terms of heat transfer and in many labs, because of this, we know that heated stages / workstations have the temperature turned up below 37 degrees which is, without being measured, a risk. We should measure in every individual situation the temperature that actually exists within the drop and not make some assumptions about what will happen, should we change the temperature of the heated surface.
So the choice of embryo culture dish can make a big difference in terms of heat transfer as described here, but it can also have an impact – as we’ll discuss later – on osmolality and the prevention of toxins from the environment invading the culture media drop and affecting embryo development.
Media pH and CO2 concentration
We know from many publications and from our own experience that pH can profoundly affect the development of embryos and the quality of eggs.
Two areas where we have to take particular note are:
- When eggs have been denuded; they have failed to have the ability to regulate their internal pH.
- And also after they’re warmed following cryopreservation; the embryos lose the ability to regulate their internal pH for a number of hours.
These two areas need to be particularly adhered to to maintain a stable pH.
But other effects can be seen and have been reported. For example, egg mitochondrial localization development competence is affected if pH is changed, egg metabolism, embryo metabolic activity, intracellular organization, and overall embryo development can be affected by changes in pH. The rate of blastomere division, fragmentation rates, and overall embryo quality are going to be affected by pH and, therefore, it’s critical that we keep it stable and within a narrow range as the embryo’s developing.
pH is set by the percentage of CO2 that we give the media but, in most commercially available media, the optimal external pH for human embryo development is not truly determined. We know that the internal pH of embryos is around 7.1 and so, to avoid acidification, the lower level of pH for the media is generally set at 7.2. There are some labs that will go below this pH but the recommendation from us for commercially available media is that the lower limit is set at 7.2.
There are studies that suggest that you get poorer embryo development of a pH greater than 7.4 so most commercially available media will tell you that the pH should be set at 7.3 plus or minus 0.1. Therefore, pH 7.2 to 7.4.
We know that the external pH is a function of the bicarbonate in the media. The bicarbonate concentration is set by each manufacturer making media. Therefore, in the lab the thing that we can do to change the pH is obviously change the concentration of CO2. Indeed, a question often asked is ‘what should I set my CO2 concentration to be to give the correct pH within the media?’
One of the things we need to bear in mind, though, is that when you’re measuring pH and when you’re getting your dishes ready for culture of embryos you need to give them enough time for the dishes and the media to reach equilibrium. This takes a number of hours so there are three different points along the path of getting pH to a stable level; equilibration, reaching the set point, and then stabilization itself (where you see variation affected by opening of the incubators etc).
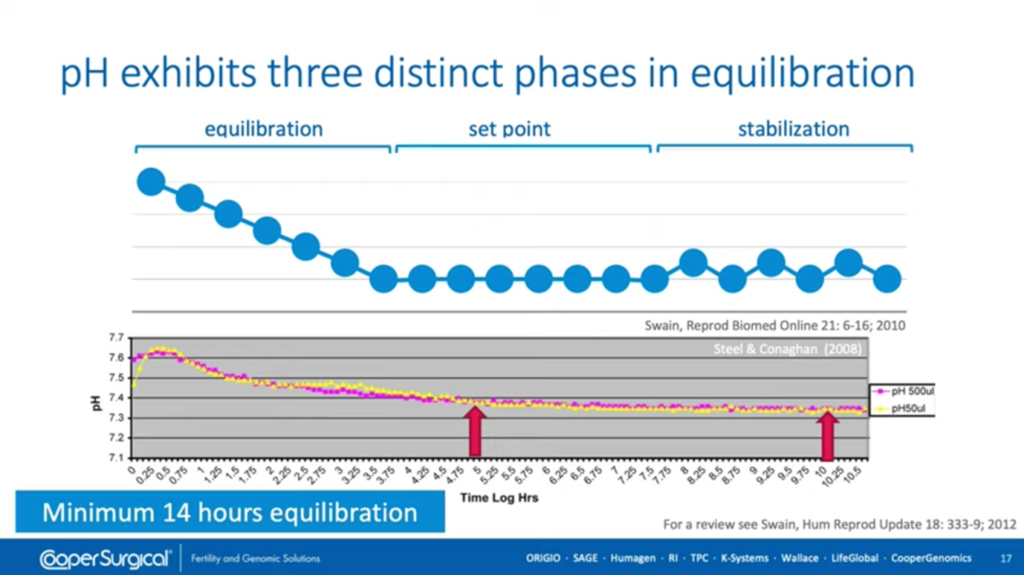
The graph on the bottom shows the time log in hours to reach that point and it’s generally longer than people expect. Nowadays, we recommend in most laboratories that you have a minimum of a 14 hours for your dishes to equilibrate. This is variable depending on the drop sizes you use, the amount of oil, and the type of oil that you use but, in general, longer equilibration is better and certainly most labs would give overnight equilibration which is generally adequate for reaching the correct pH level.
There are several factors which affect pH of the culture media. Go back to where the question is asked, ‘what CO2 concentration should I use to get my pH correct in my media?’ It’s a very difficult question to answer because of these variables that exist.
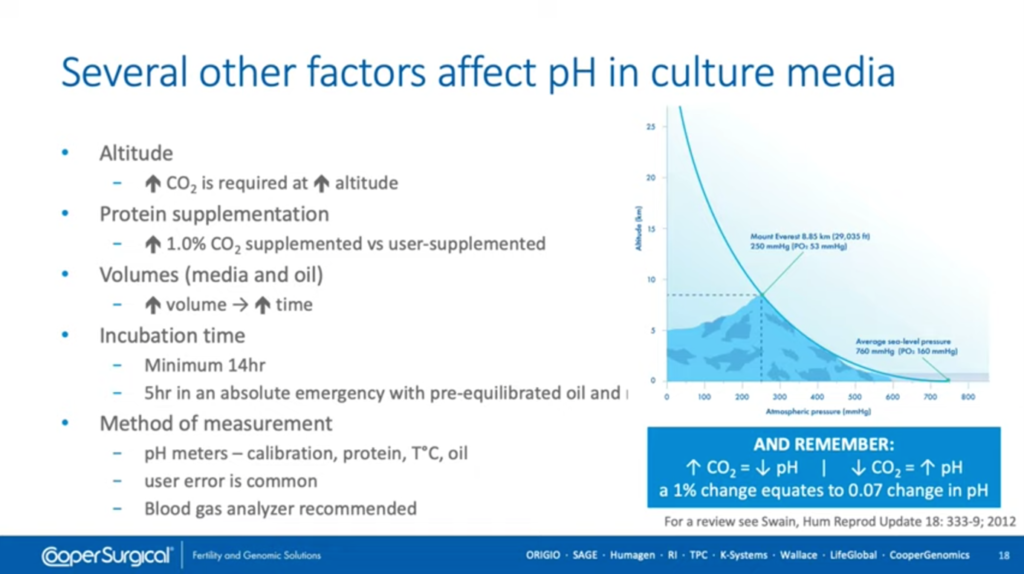
Altitude plays a major part in the CO2 concentration that’s needed. As you increase the altitude, you increase the CO2 concentration that’s needed to give the correct pH. That’s due to partial pressure of gases at different altitudes.
Whether a lab supplements their own media or buys pre-supplemented media from the manufacturer will have an impact on the amount of CO2 that’s needed to give the correct pH within the media.
The volume of media and oil that we use. Increases in volume of oil means increased equilibration time and therefore, as we’ve said in the previous slide, we recommend a minimum of 14 hours for incubation to get the dishes to be equilibrated. But, in an absolute emergency, if you have pre-equilibrated oil and media available in your lab then you can make dishes in an emergency and give them a few hours to equilibrate. So when you’re using pre-equilibrated material to make your dishes then the time obviously comes down, but the recommendation is to do it overnight.
How do we measure pH? Well, of course, this has been a conundrum for many labs over many years. There are pH meters that are designed for IVF labs but they require calibration. The protein concentration can have an impact, as can the temperature that you do the measurements and also the presence of oil.
We also see, when we go and check on pH, that some of these issues are not dealt with in a perfect manner and therefore the measurement of pH is often not accurate. I’ve found – and certainly others have found – that when they travel and measure pH in situ, a blood gas analyser generally gives better, more reproducible and more accurate results in the situation of looking at the pH in the drops under oil that the embryos would see rather than a surrogate tube that’s kept in the incubator where there is a risk that pH changes very rapidly once you remove it from the incubator.
So, factors affecting the pH in your media:
- The length of calibration time
- The frequency that you open the doors (which not only affects pH but other factors as well and therefore should be kept to an absolute minimum)
- The type of CO2 sensor
- Where you place your air conditioning outlets
- Whether there are temperature changes within the incubator (so obviously the type of incubator that you use)
- Whether there’s contamination present or not
- The altitude of the clinic can have a major impact
- Whether you use humidified or non-humidified incubators and the relative humidity in the lab can also affect pH by having an impact on evaporation
Micro-Droplets And Optimised Embryo Culture
Most labs now would use micro drop culture. It was worked out a few years ago in some publications (as you can see at the bottom of the slide here) that micro drop culture works very well for embryo culture.
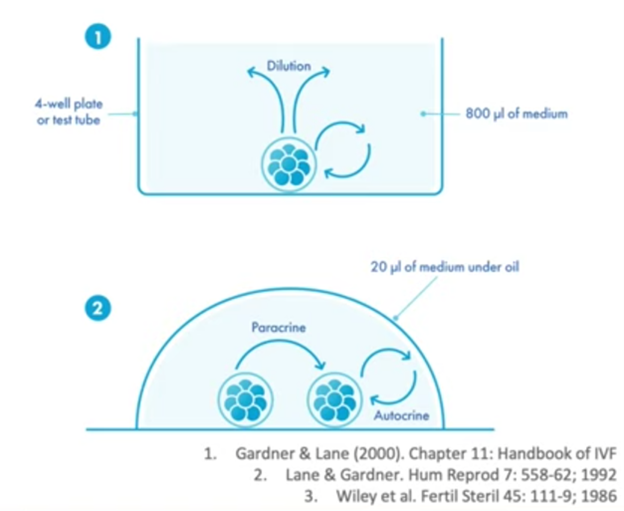
Decreasing the incubation volume increased embryo viability in the mouse. And, also in the mouse, the cleavage stage embryos and blastocyst formation increased with reduced volume. So this has led to most laboratories around the world culturing now in micro drops
under oil and this is a very successful technique if you’re very careful in how you prepare the dishes and also the volumes of media and oil that you use in your culture.
Osmolality’s Effect On Embryo Development
We also want to touch on osmolality, the control of which is critical to embryo development. This graph here, produced from Jason Swain’s paper in 2012, gives a clear demonstration of how a little change in osmolality can have a major impact on the development and the competency of your embryos.
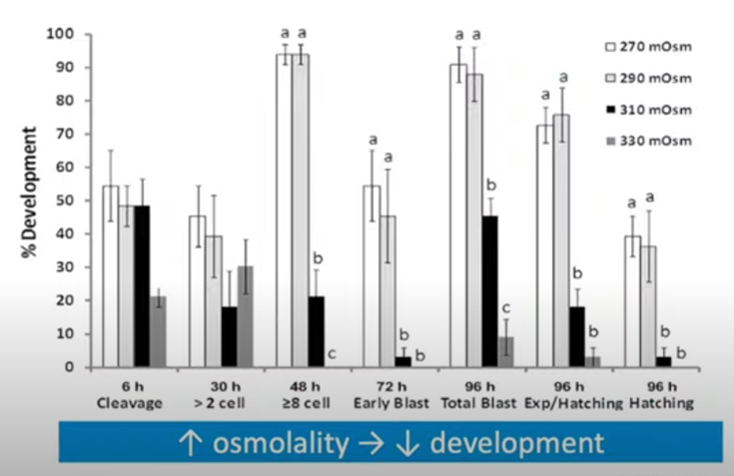
From an early cleavage stage, right through to blastocyst and hatching blastocyst, a relatively small increase in osmolality can have a major impact on development. Here we’re looking at changes from 270 to 330 milliosmoles per kilogram having a significant impact on each of these different stages, so something we should very much take care of.
How do we control osmolality? Well, generally we do it by the oil overlay in our media.
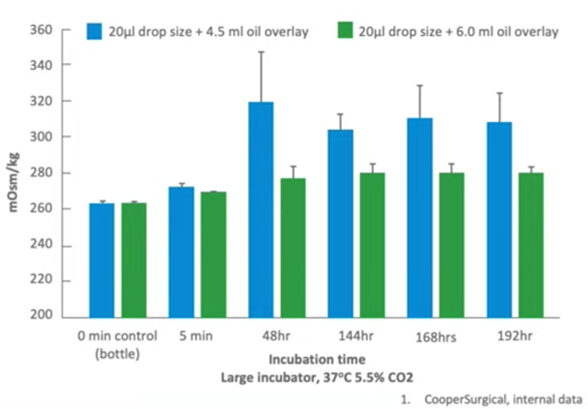
This is an in-house study which was done by CooperSurgical looking at just a very small increase in oil from 4.5 milliliters to 6 milliliters in an overlay and looking at what effect that has on osmolality over time. Of course, as we said right at the beginning, we’re now looking to culture embryos for an extended period of time, which puts increasing pressure on those drops to remain in a perfect condition for embryo development.
We can see here that when you use 4.5 mls of oil as an overlay, within a very short period of time you start to see changes in osmolality when you use a 20 microlitre drop. Just an increase of 1.5 mls to 6 millilitres resolves most of this problem. So the mantra should be (in our laboratory) that we should use a maximal amount of oil, not a minimum to ensure that osmolality doesn’t change over time.
How To Prepare Dishes For Optimal Embryo Culture
Our dishes have to sustain osmolality for up to eight days (mostly five, six or seven days but it can be stretched further).
So what influence does the oil have?
- We can use different techniques to make the dishes, whether we use an underlay or an overlay procedure
- What volume we use
- The type of oil we use (the light versus heavy oil changes in viscosity)
Whether we use a humidified or a dry incubator will have an impact. There are three types, generally, on the market; those large humidified box incubators, a benchtop incubator which is humidified, and also now a current trend to move towards benchtop incubators which are dry. If these are the ones that are used in your laboratory then it is even more critical that the correct volume of oil and the right type of oil is used.
So does the type of dish influence osmolality stability? It does. Cell culture dishes can have an impact and those that have pre-formed wells for embryo culture seem to do better. From the studies that have been done, if you underlay your drops, these are certainly the best way to prepare dishes. So you put your drops in the dish, you put oil over the top, you remove those drops and then you replace them with new drops straight under the oil so they give the least amount of risk of evaporation.
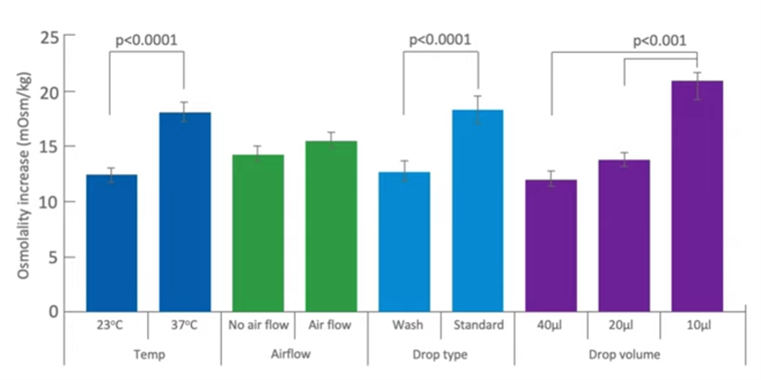
Here’s a graphical representation of that. Different factors that can affect the osmolality in the way that you make the dishes, from temperature, air flow, the drop volume, drop type etc. It’s really very easy to see that if you can keep the conditions at a low temperature with low airflow, you underlay the drops and you use a reasonably good size drop, you will minimize the risk of changes in osmolality in the in the preparation of your dishes.
Drop volume is certainly one that should be taken into account significantly. Labs, generally, are moving to smaller and smaller drops, and the smaller the drop becomes, the more risk there is to evaporation as they’re being made. Generally, 30 to 50 microlitres is recommended for embryo culture; this gives all the benefits without many of the risks.
So, in terms of osmolality, we need to create an environment that gives the embryos chance to develop. That volume is not determined – we don’t know optimally what it is. There are some labs that use very small drops and they seem to get it to work but it may not work everywhere. We know that 20 to 50 microlitre volumes is more typical and generally safer. The drop size may affect longer term changes in osmolality; once the osmolality has started to rise it is unlikely to go down and will only get worse.
So when we’re making dishes (and this may seem very simple when we’re discussing optimizing systems) but dish preparation is critical when we’re trying to culture our embryos for significant numbers of days (five, six or seven).
- Generally make one dish at a time
- Use dishes that are specifically designed for IVF and embryo culture
- Minimize the airflow
- Try to work at room temperature with unheated stages and workstations
- Underlay drops where possible. This extends the time of making dishes but it’s certainly the best way and the lowest-risk way of producing droplets
- Use the maximum amount of oil that the dishes will allow without causing oil sealing of the lid on the dish
- And the type of oil should be assessed to use the best that is available for you in your condition in your laboratory
The Effect of Temperature on Optimising Embryo Culture Systems
The temperature is as critical as pH and needs to be controlled throughout the IVF procedure. There were some suggestions that temperatures below 37 degrees may give better outcomes but the Cochrane review that was done generally indicates that that’s not the case. Really, keeping the temperature as close to 37 and stable as possible is beneficial for long-term embryo culture and it’s certainly true that we should not increase the temperature above 37 degrees. This is detrimental to embryo development.
So, again, close attention to temperature in your laboratory is important and needs to be monitored in the situation if possible, using temperature probes into the droplets to ensure that they remain constant and at the right temperature throughout embryo development.
The Effect of Oxygen Tension on Optimising Embryo Culture Systems
There’s been many publications in this area. If you look at all of the outcomes relating to live births, pregnancy rates, blastulation rates etc, they’re all superior when you culture embryos in low oxygen tension (5% oxygen as opposed to atmospheric 21%).
So, if you can, culture your embryos in low oxygen to give the best outcomes and the best quality embryos, to give the best implantation rates and your patients the best chance of success going forward.
The Role of Oil In IVF Culture
Oil is important in IVF culture because:
- it minimizes the changes in pH. If the pH does change the embryos experience stress and will not perform as well as they might.
- It also prevents change in osmolality and, again, we saw that a very small increase in osmolality can have a very significant detrimental effect on embryo development.
- We can reduce the risk of temperature fluctuations.
- It protects the samples from your embryos from the outside world – particularly from VOCs which are most prevalent in the gas supply to your incubators but also present in plasticware and the general lab environment (the people etc that are in the laboratory). We don’t have time to talk about air quality today – it’s a whole other area we could potentially talk about – but for the moment we’ve been looking at pH osmolality and temperature.
As said previously, we should use the maximum amount of oil in our culture dishes, without running the risk of oil sealing the lids.
Oil for embryo culture generally goes by two names; mineral or paraffin oil.
Are they all the same? Well, in terms of their name, if you look at the U.S. pharmacopoeia it’s called mineral oil, in European it’s called paraffin oil. In essence, they’re the same thing; they have the same chemical abstract, which contains a mixture of straight chain hydrocarbons and cyclic hydrocarbons. They’re fully saturated. So, in terms of their chemical makeup, they are [the same] but in terms of testing and in the viscosity there are some differences.
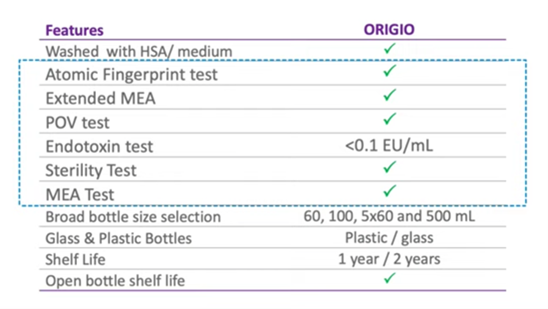
So this is an overview of the of the testing that’s taking place with ORIGIO mineral oil and, as you can see, it’s a very comprehensive testing regime that’s very rigorous and needs to be so because embryos are going to be cultured for long periods of time and we certainly need the oil to be of the highest quality.
Of course, we know in the past there have been problems with oil toxicity, and this is, of course, something we need to avoid so we need to use oil that’s tested to the highest possible standards to give us the least risk of having an impact on our embryos.
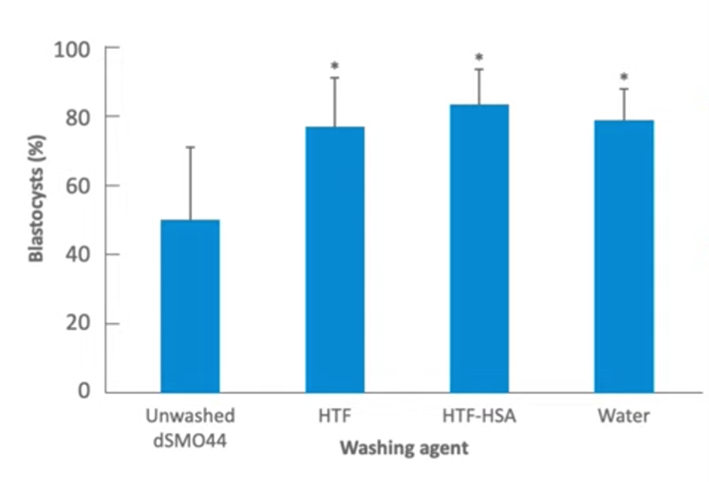
This paper by Dean Morbeck in 2010 showed very nicely that, when we’re using oil it should certainly be washed, either with water or with media, or media containing HSA. In his experiment, when the media that contained HSA was used to wash the oil prior to use, that gave the best results. So, where you can, use washed oil for embryo culture.
Summary
We need to achieve optimal results for our patients to give them the best possible chance to have a healthy, normal singleton birth from all of the cycles that we perform, where possible. To do that, the IVF lab and the embryologists need to have great attention to detail. We need to adhere to the protocols as much as possible and follow SOPs, and quality control / quality insurance is vital.
IVF culture media is an integral part of the embryo culture system but it’s not the only part and therefore we must optimize other factors. Every laboratory has to work out the optimal conditions for itself. We need to control and monitor pH, temperature and osmolality as much as possible and this needs to be monitored and maintained in a narrow range to give the least amount of stress to embryos as possible, again to give them the best chance of reaching a healthy, normal stage prior to transfer.
The choice and the use of oil is critical; we’ve discussed dish making and why the dishes need to be made in in the right way, one at a time, using the correct technique and the right amount of oil.
And, of course, consumables and equipment in the laboratory need to be of the highest quality. They need to be tested regularly to make sure they remain within the specification, maintained where appropriate and on a regular basis, and records kept for that.
So that’s a brief summary of what we need to do in the laboratory to optimize our conditions.
About the Presenter
After completing his PhD in Molecular Immunology in 1989, Steve Levett obtained a position as Postdoctoral Fellow at York University. He then took up his first embryology role in the early 1990s at St. James’s University Hospital in Leeds, UK. He became Lab Manager soon after and then went on to manage other embryology labs in the UK and Canada, in the public and private sector.
From 2006-2014 Steve worked for Wallace, then part of Smiths Medical, becoming Global Business Director in 2010. He then joined Ferring in 2014 as UK National Business Manager for Obstetrics and Fertility and 18 months later joined Life Global as VP International Sales.
Steve has a keen interest in Lab optimization, QC procedures, optimization of results and supporting embryologists. His research interest has been in fertility preservation since working with Prof. Roger Gosden in Leeds. Steve Levett is Director of Clinical Application at CooperSurgical Fertility and Genomic Solutions.
Useful links
If you would like to explore any of the products / references mentioned in this article, please click on the links listed below:

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
