In this webinar, David Chrimes, PhD discusses the frequency of aneuploidy in miscarriage, particularly in relation to maternal age, and shares evidence for utilization of PGT-A for miscarriage reduction.
How PGT-A Reduces the Chance of Miscarriage
In this webinar, CooperGenomics Business Development Director, David Chrimes PhD discusses the frequency of aneuploidy and miscarriage, particularly in relation to maternal age, and shares evidence for how the utilization of PGT-A reduces the chances of miscarriage in general.
Today we’ll be covering four major topics:
- An overview of how miscarriage and maternal age are linked
- A look at the impact of miscarriage from a psychological point of view for the patients involved
- We will then quickly touch on PGT, reminding ourselves of the technology and explaining why this plays such an important part in in the reduction of the chance of miscarriage
- And then we’ll take us from the theory of why PGT-A is important for the reduction of miscarriage to the point at which, actually, there is significant evidence now in how this is actually seen in the field and in clinics.
Miscarriage and Maternal Age
One thing we’ve known for many years is that 50-70% of pregnancies that result in a spontaneous loss have some kind of cytogenetic abnormality. When we say ‘cytogenetic’, what we really mean is ‘chromosomal abnormality’.
Those chromosomal abnormalities might be:
Autosomal Trisomy: A human genome is made up of 24 pairs of chromosomes – you have the autosome and then you have your sex chromosomes (so if you’re a female you have XX, if you’re male, XY). Your autosomal chromosomes are the ones that are shared between both males and females and they account for 60% of those spontaneous losses of pregnancy.
Monosomy X (also known as Turner syndrome): Not all Turner syndrome make it to live birth and it seems that, if you have a Monosomy X, then a lot of those will also miscarry.
Polyploidy: This is where you have more or less that the exact number of chromosomes. That could be a haploidy (where you only have one copy of the genome, not two) or you can have three or four copies.
These are all detectable. They’re even detectable in the embryos, so we can test all of these conditions before we put the embryo back in IVF. We’ve been doing this for many years and, what we have now established (as we now do day five, day six, or even day seven trophectoderm biopsy) is that it is proving to be very robust.
Of those embryos available for testing (i.e. those who have made it to day five, six or seven), even in egg donors 25% of those embryos are aneuploid. Yes, the majority are euploid, but you still have 25% being aneuploid.
Then you have a curve or a line which we’ve seen many times over the years, where, as the maternal age increases, you get a dramatic drop-off in the number of euploid embryos available and a corresponding large increase in the number of aneuploid embryos. Really, the tipping point is around the maternal age of around 35; under 35 and half the embryo are euploid, over 35 less than half are euploid.
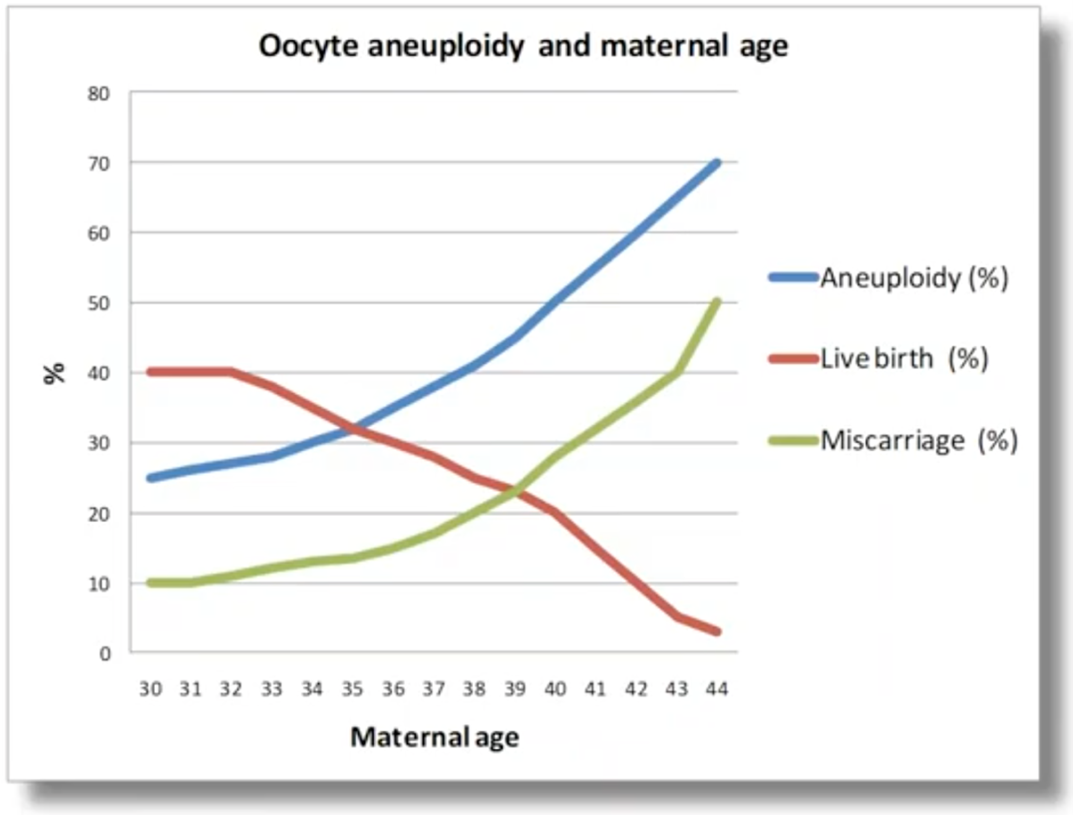
What this means is that, if we look at how pregnancies progress, as maternal age increases, we have this classic chart (above). As the maternal age increases, the amount of aneuploidy sees a massive increase. This is driving the miscarriage rate to follow the same trend as the aneuploidy (as I’ve just explained, aneuploidy is the leading cause of miscarriage). And what that results in, is a massive reduction in the live birth rate as the maternal age increases; particularly once we reach past the age of 35.
What we are also seeing is an increase in maternal age of patients. As of 2017 (and I’m sure the trend has continued and, in fact, probably increased) over half the patients being treated in IVF have a maternal age of over 35 – very much in the ‘at-risk’ group when it comes to elevated risk of miscarriage.
The Impact of Miscarriage
Because we know that miscarriage increases with age, we can counsel patients accordingly. We can tell them to perhaps even expect a high chance of failure and a higher risk of miscarriage. At some level we are fulfilling our duty by doing this. However, it is very hard to understand, as an individual, what impact miscarriage may have.
An initial study,1 published in 2018, showed that miscarriage had a huge psychological impact on many women. They found that, in 15% of women, they can suffer deep depression lasting up to four months after that miscarriage. That depression and feeling of loss was comparable in nature and intensity to grief reactions from people suffering other types of major loss. They found that four-in-ten women experienced post-traumatic stress at some level. So, this is a huge amount of psychological impact with each miscarriage.
This work was then followed up with a report2 focusing particularly on the post-traumatic stress, which found that, when they analyzed interviewed 1,500 women, a huge number of them – four out of ten – had suffered post-traumatic stress, anxiety and depression after miscarriage.
Yes, it declines over time, but it can remain at clinically important levels for at least nine months. So, if we’re considering a patient who’s had an embryo transfer, they got pregnant, their hopes are soaring, but they then have a miscarriage, that miscarriage will live with them for many, many months potentially. It may delay or, in fact, stop them having any future transfers just to avoid the risk of the heartache that a miscarriage can cause.
PGT-A Overview
To be completely open and honest, this is why PGT-A was first considered to be a potential tool in embryo screening. If we could find the embryos that carried these autosomal or sex chromosome abnormalities before you transferred them, then you could theoretically reduce a lot of the embryo transfers that would ultimately lead to a failure to implant or miscarriage.
And so PGT-A is a technology – a screening test – that assesses the embryo’s chromosomal health. It looks at the embryo and tries to establish if it has the expected amount of chromosomal content – your genome. It then informs the transfer decision, so it identifies the embryos most likely to lead to a healthy pregnancy. It cannot guarantee a healthy pregnancy, but it can increase the chances of their embryo transfer resulting in a healthy pregnancy. By doing that, we can improve the IVF outcomes for patients.
So how does it work? Well, you should have a euploid embryo. A euploid embryo is an embryo which contains the correct number of chromosomes and these, we know, have the highest likelihood of success. As I was stating earlier, both males and females have chromosome pairs 1 to 22 (your autosomal chromosomes) and then, if you’re a female, you have two copies of X, and if you’re a male, you have one X and one Y. So, what we’re testing for is to find the embryos which look good (euploid).
What we also want to test for – and try to screen out – are the embryos which contain aneuploidy and aneuploid chromosomes.
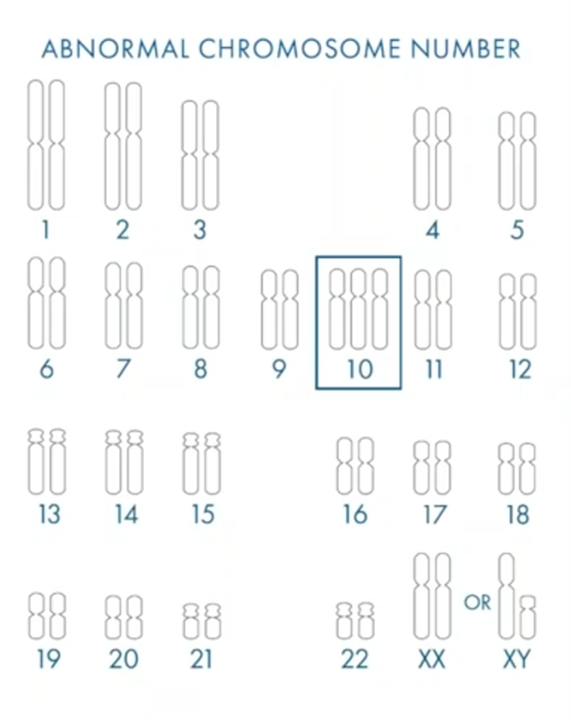
In this case (above), we’ve highlighted chromosome 10 as trisomy. There has been no recorded live birth of anybody in the world with a trisomy chromosome 10. What this tells us is that, if you find an embryo with the trisomy 10, the chances of that actually resulting in a successful pregnancy are incredibly low and so we’re trying to ensure that these embryos are found before transfer.
But what we know not just about chromosome 10 but any chromosomal aneuploidy in an embryo, is that the most likely outcome is either a failed implantation (so you don’t even get pregnant) or, if you are fortunate enough to get pregnant, then the vast majority will miscarry. Some of those can be quite late miscarriages, depending on the chromosomes. Or, if it does go to term and a live birth, then that child is at a much-increased risk of some form of genetic disease.
We do have the potential to find mosaic embryos, where there is a mixture of both euploid and aneuploid cells. We know that mosaic embryos implant less and miscarry more, but they can sometimes lead to live birth. So PGT-A will also identify these embryos. (If you want to learn more about mosaicism, then please find the webinar on mosaicism3. It’s a great webinar and it will tell you more about mosaic embryos and the outcome of those mosaic embryos.)
Obviously, everybody would like to reduce miscarriage where they can.
Evidence for PGT-A Reducing the Chances of Miscarriage
There have been many clinical trials over the years which have used an array of technology, and most of them have shown a reduction in miscarriage. What some people have done to try to bulk the numbers is do a meta-analysis; that’s where you group all the papers and pull all the data together. The ones which use today’s technology or closest to4,5, all show a decrease in miscarriage rate as well as an expected increase in implantation rate and clinical pregnancy rate.
A lot of IVF data is generated through observational databases across groups but also within a single clinic. This single clinic study6 included [patients of] advanced maternal age and what they found is a clinical miscarriage rate of under 10% in patients who are over the average age of 38.
But the other thing was that miscarriage was not affected by embryo morphology in their study. A lot of work and effort is put into grading embryos on morphology with the aim of using that to predict the outcome of the embryo and, for sure, there is some variance in that. But the key driver in this study showed that it was not the morphology, but the genomic competence – or the euploid status – of these embryos which was most critical in reducing miscarriage.
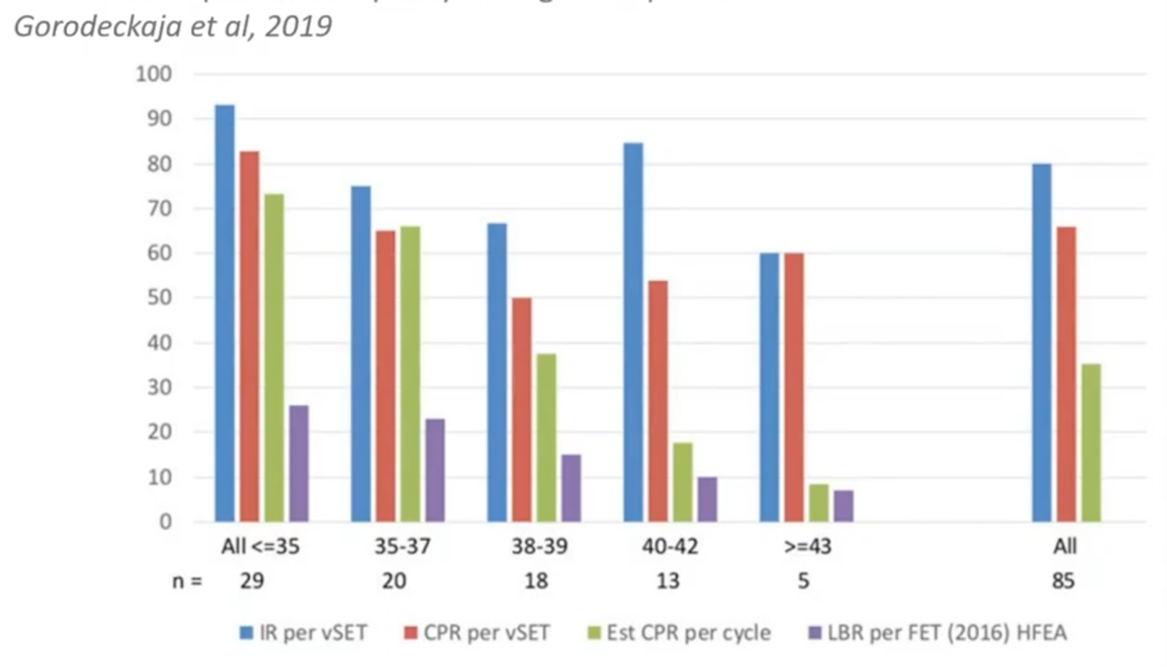
This (above) is more internal observational data from a clinic. They offered all their patients PGT-A that wanted it and found that the expected implantation rate stayed very high across all patients’ age ranges if you found a euploid. In addition, their sustained pregnancy rate also remained very high, showing a very low miscarriage rate.
Of course, if you do PGT-A, you are reducing the number of embryos available for transfer because you’re taking those which are aneuploid out of the ‘pool’. So, what you actually find as the patient ages (and this would be true for any patient, whether you do PGT-A or not) is that you do start to reduce the number of live-births-per-cycle-started. That that is just a natural fact of the patient aging and nothing to do with PGT-A at all.
This is another paper7 where they’ve tracked the performance of PGT-A over four years-worth of testing. What they found is that the PGT-A group had a miscarriage rate of less than half of their control group. It dropped overall (across all patients) to 4.4% versus their untested patients’ rate of 12.9%. This was a statistically significant finding. And, just to be clear, they scored their spontaneous abortion rate as the ‘loss of cardiac activity’, so the pregnancy had to get at least as far as detectable heartbeat.
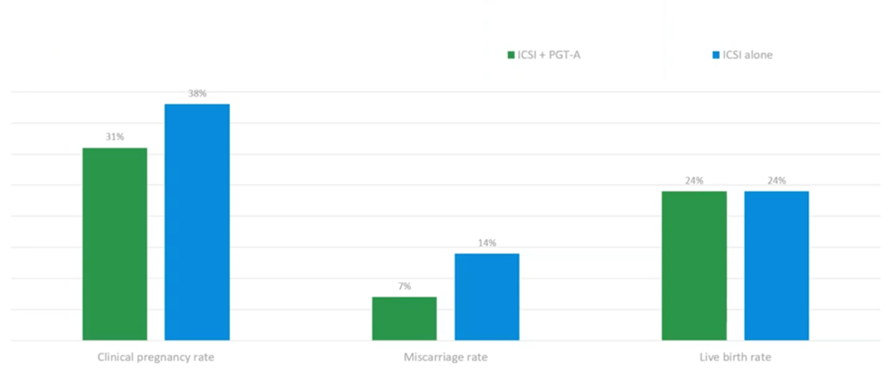
This (above) is actually a clinical trial. It was run on polar bodies, taking polar body 1 and 2. This is just looking at the maternal contribution in advanced maternal age women. What they found was no reduction in cumulative live-birth-rate-per-cycle-started. If you did PGT-A, what they did find is a halving of the miscarriage rate. This doesn’t even take into account the male factor. So, again, a significant finding. If you do PGT-A, even on the polar bodies (which is the most expensive way of doing PGT-A), by excluding those meiotic errors from the oocyte you can actually halve your miscarriage rate. This was a secondary finding in the clinical trial, and it was noted as being significant for the management of patients.
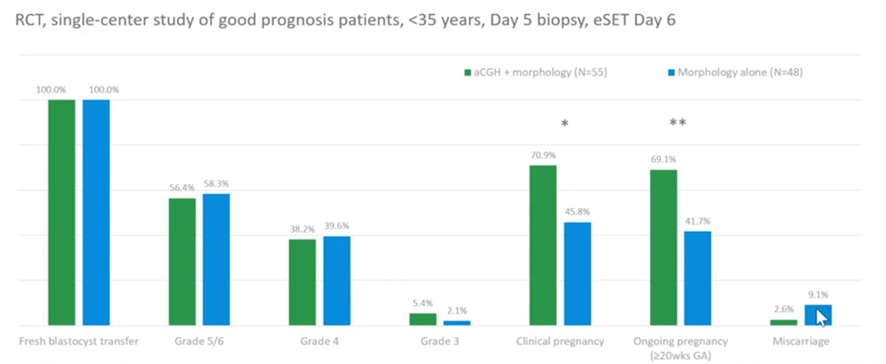
This (above) is another RCT (controlled trial) but this is done in a single center rather than multiple centers. Yes, it was a small group but, again, what they found is (and bearing in mind these were patients who are under 35 – so your highest prognosis, lowest chance of miscarriage) that in their control group they had a miscarriage rate of 9%; if they did PGT-A that dropped right down to 2.6%. Again, a massive reduction in miscarriages if you screen out the embryos which are most likely to result in miscarriage due to being aneuploid before you transfer them.
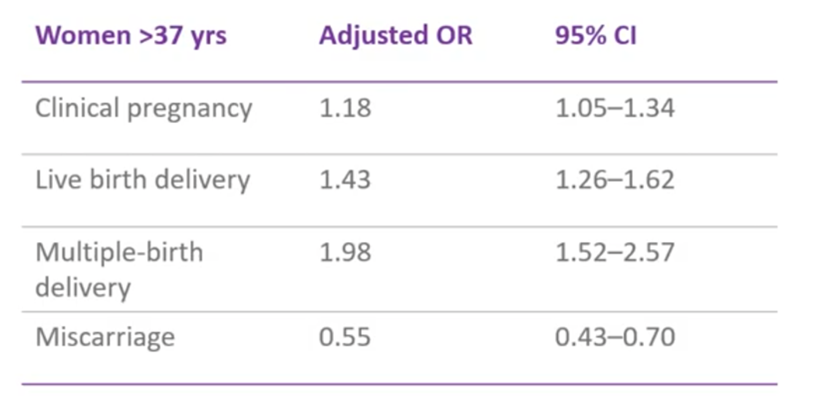
This (above) is a big, retrospective study. It involved climbing back through their data, so it’s not a full clinical trial, but it was a retrospective cohort study, matching cohorts where possible. What they found was that, for women above 37 years of age, PGT-A was associated with the decreased odds of miscarriage i.e. their chances of having a miscarriage were much reduced if those patients had had PGT-A. By decreasing miscarriage, of course, we increase our odds of a live birth. In this particular study, they were still doing multiple embryo transfers (in the UK and many countries now we still try to do single embryo transfer, however they were putting back multiple embryos which have been screened by PGT-A) and they did find an increase in multiple live births.
SART Data
SART is the U.S. database for the Society for Assisted Reproductive Technology. They gather data from approximately 90-95% of all the IVF clinics in the USA and accumulate data over all cycles, all clinics and all years.
We have looked at this data from 2014 onwards. We’ve chosen 2014 specifically because the vast majority of these were in trophectoderm biopsy (so very similar to what we would practice today) and they were also – the vast majority – run on the newer technologies of NGS (so as comparable to what we’re running today as we could find).
What we found with this huge data set (and this was over nearly 450,000 cycles, of which 120,000 were PGT-A) is that PGT-A massively reduces your miscarriage rate as the maternal age increases.
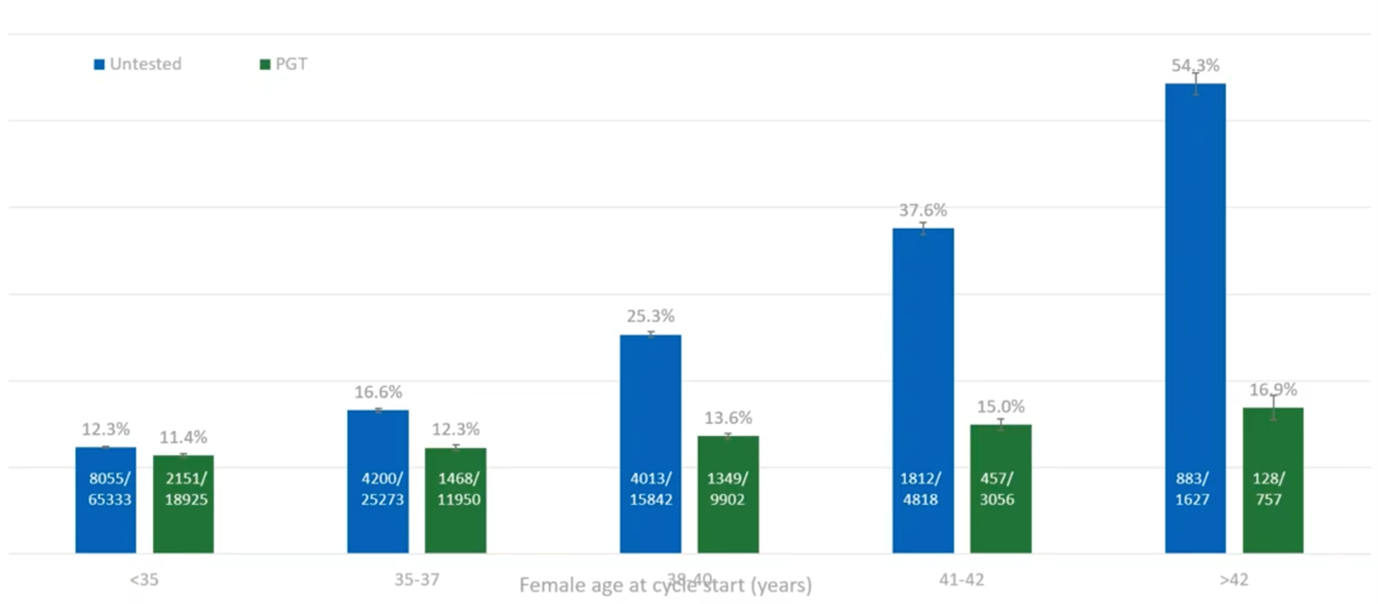
The blue bars (above) – the untested – we have the ‘expected’ and ‘seen’ in both natural and IVF, increase in miscarriage rate as the maternal age increases. If we test before we transfer those embryos, we managed to maintain a pretty low level of miscarriage. Yes, it increases slightly with age, but we’re maintaining a level which is comparable to the under 35s. This is a great message; if you have PGT-A, you can basically reduce your risk of miscarriage back down to the background level of the under 35s.
This data also shows that what, ultimately, a patient wants when they have IVF is a live birth, clearly. They also want to go through as few procedures and embryo transfers as necessary to achieve that live birth.
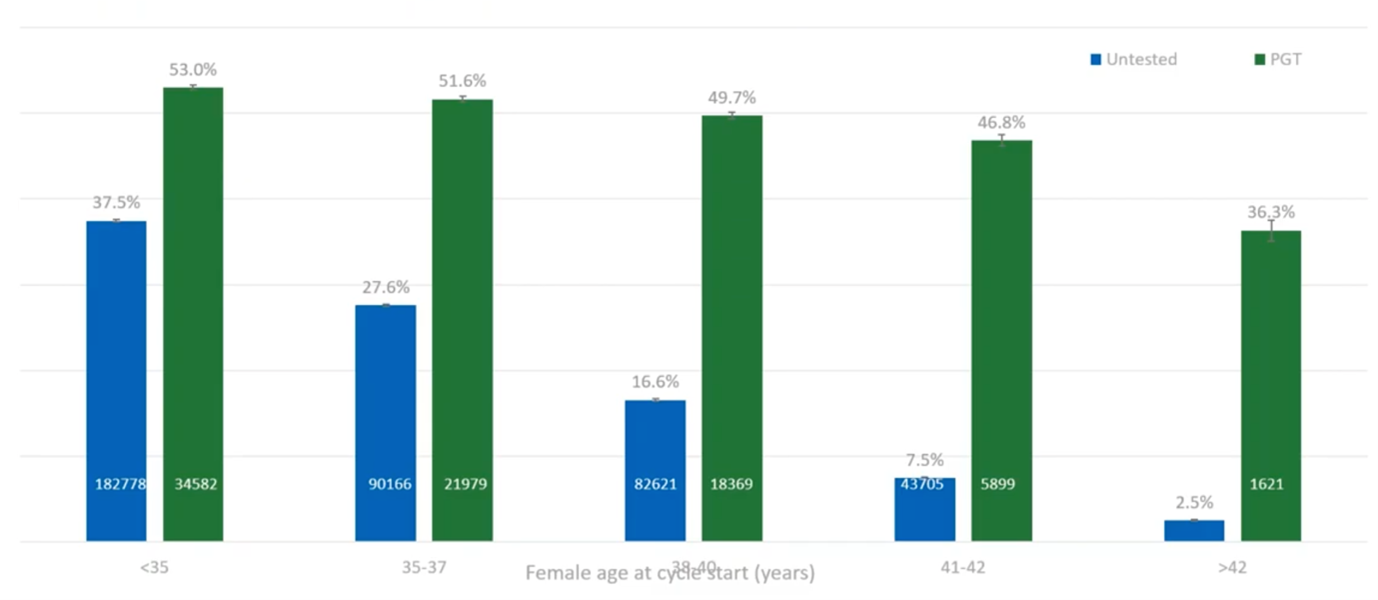
What we have here (above) is showing that, basically, if you do PGT-A (at least with the under-42s) you’ve got around a 50% chance of a live birth per embryo transfer.
If you don’t do PGT-A, by the time a patient reaches 38, basically one in six embryos will result in a live birth, so you’re doing five or six embryo transfers for every live birth. If you do PGT-A, you’re only doing two embryo transfers per live birth, so a huge reduction in the number of transfers required and also a huge reduction in the number of miscarriages that you would see.
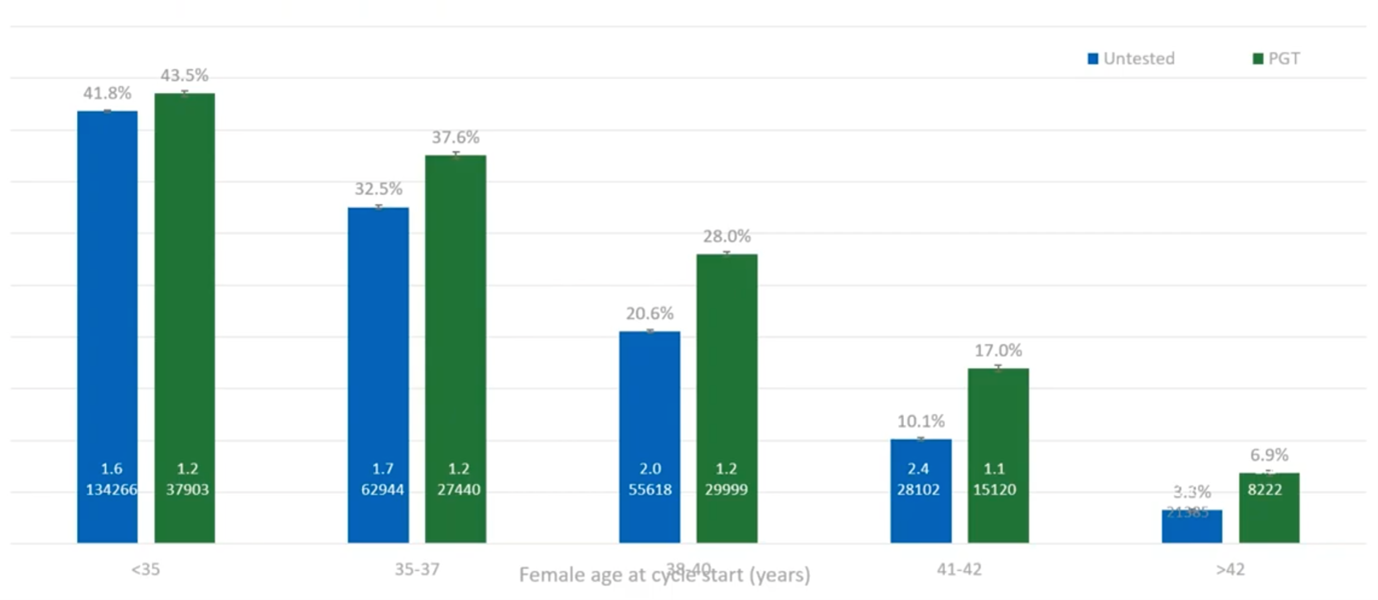
By avoiding these miscarriages – by avoiding the trauma of failed transfers – patients with PGT-A have a higher cumulative live-birth-rate-per-cycle-started. We believe this is because they are using only the good embryos, they are getting them earlier and they’re avoiding the traumatic events which cause people to stop IVF and pull out. So, if you have PGT-A, you’re only having the good embryos transferred, you have less psychological impact of failed transfers, you push through and you get this increase in live birth per retrieval. And that, again, is a great message for patients.
The other aspect that PGT-A can lead (which is not just miscarriage but one of the risks of any pregnancy) is preterm deliveries. A preterm delivery puts a huge burden both medically (on the hospitals) but also a huge increase in risk of that child succumbing before they achieve their potential. What we have found with PGT-A is that there is a reduction in these preterm deliveries, the embryo is going in better, they are generally single embryo transfer (so if you have twins, you have a higher risk of a preterm delivery as well). So PGT-A not only reduces miscarriage, but it can also reduce the likelihood of preterm deliveries and all of the health complications that that can bring.
Summary
- Miscarriage is highly traumatic. Most of the studies look at the traumatic impact of miscarriage in women. However, it is noted in many of the studies that men also suffer (quite often silently) during a miscarriage and should not be forgotten.
- We know that the major driver of miscarriage is chromosome aneuploidy.
- We know that PGT-A can detect those aneuploidies before you do the embryo transfer and this has now been shown repeatedly across many studies and across many groups.
- PGT-A can mitigate against this age-related miscarriage risk (it can’t eliminate it as there are other causes of miscarriage beyond chromosomal abnormalities).
- Overall, PGT-A has the power to reduce the emotional trauma and increase the chances of a successful live birth per cycle started for patients undergoing IVF. This is particularly true for older patients who are at this increased risk of miscarriage and the increased risk of multiple miscarriages without screening.
About the Presenter: David Chrimes PhD
Dr Chrimes is a PhD molecular geneticist with 14 yrs. experience in molecular diagnostics.
In 2016 Dr Chrimes started at Genesis Genetics to help drive advances in their embryo testing and, after the acquisition in April 2016 of Genesis Genetics by Cooper Surgical Industries (USA), Dr Chrimes took on a global role as Director of Global Genomics Business Development.
This role ensures Dr Chrimes is both driving innovation in genomics testing and also gaining regular insights to the challenges faced by IVF clinics today.
Find out more about David Chrimes PhD.
Additional References
The following are mentioned throughout the text above and/or are particularly relevant to it. To find out more about each item, please click the link.
- Reference
- 1 FIGO (The International Federation of Gynecology and Obstetrics), What is the psychological impact of miscarriage?, 2018
- 2 Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open, 2016
- 3 CooperSurgical Mosaicism: Everything you Need to Know webinar
- 4 Chen et al. Can Comprehensive Chromosome Screening Technology Improve IVF/ICSI Outcomes? A Meta-Analysis, 2015
- 5 Dahdouh et al., Comprehensive chromosome screening improves embryo selection: a meta-analysis, 2015
- 6 Gonzalez et al., Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients, 2019
- 7 Anderson et al., Clinical benefits of preimplantation genetic testing for aneuploidy (PGT-A) for all in vitro fertilization treatment cycles, 2020
- Genomics
- Procedures

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
