Olga Razina, MSc offers tips and tricks for successful Vitrification.
Tips and tricks for successful vitrification
The focus of this webinar is on tips and tricks for successful vitrification protocols. We will cover many different aspects of this including:
- Basic principles of cryopreservation
- How to manage expectations
- The education processes
- Evaluation of results using key performance indicators implemented in the lab
- Traceability and witnessing – these are one of the most important topics nowadays, especially because of the increasing number of PGT cycles and the movement of samples from clinic to clinic
- Tips and tricks which can help you to improve results
- Summary of key points
The process
Cryopreservation
Cryopreservation is a process which preserves cells. We do this by exposing them to high concentrations of cryoprotectants, storing them at low temperatures, and maintaining their viability.
The freezing and storage of human tissues and cells is carried out at minus 196 degrees Celsius. This is done in liquid nitrogen to preserve them for future use.
At this low temperature, there are no physiological activities. According to some publications, cells and tissues can be stored almost indefinitely. Cryopreservation is not an artificial process and can be found in vivo as well.
This is a picture of an Alaskan wood frog which can tolerate the freezing of its blood and other tissues.

Urea is accumulated in its tissues in preparation for the cold weather in winter. In response to internal ice formation, liver glycogen is converted to glucose in large quantities. Both urea and glucose act as cryoprotectants to limit the amount of ice that forms and to reduce osmotic shrinkage of cells.
Why cryopreserve?
Cryopreservation is a widely used method in modern ART. The areas of its usage have increased over the last decade due to the implementation of several new techniques.
We do cryopreservation to preserve fertility in the case of diseases, donor programs and for social reasons such as delaying fertility.
We need to freeze samples in case of unforeseen events. In some cases, embryologists are faced with a situation where a semen sample cannot be produced on the day of the pickup. Thus, we need to vitrify the oocytes for the next cycle.
We must have a very well-established vitrification procedure before we start PGT protocols in the clinic.
Of course, we also do cryopreservation to optimize results as it was shown that you can increase clinical outcomes by doing so.
Nowadays, we can cryopreserve human gametes and embryos at all pre- implantation stages of development, as well as a variant in testicular tissues.
There is some evidence in favour of using vitrification for oocyte freezing. Oocyte vitrification is now a widely used procedure in the case of absence of sperm cells on the day of pickup, for oocyte pooling, social freezing and for donor programs.
Embryos can be cryopreserved with the same outcomes by using either slow freezing or vitrification. For blastocysts, vitrification seems to be a preferable technique.
Mostly, we cryopreserve embryos and blastocysts in freeze-all cycles when we have surplus, good quality embryos and when doing PGT cycles.
Sperm cells can be cryopreserved with the same results via both methods when it comes to disease, preparation for IVF treatment or for donor programs.
Cryopreservation of testicular and ovarian tissues is an actively developing area within IVF. This is mostly done when trying to delay reproduction, in the case of disease or to alleviate menopause.
Methods of cryopreservation
There are two main methods of cryopreservation of human gametes, embryos, and tissues – slow freezing and vitrification. They have both been used in ART laboratories for the past few decades and are based on the principle of dehydration.
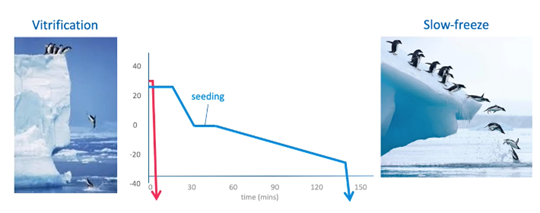
Slow freezing involves freezing of samples in a controlled, slow decrease of the temperature environment with a slow increase of osmolality around the embryos. It requires a rather expensive rate-controlled freezer. You can see the temperature profile of slow freezing in the blue line above.
Vitrification allows the solidification of the cells into a glass-like structure without forming ice crystals. It requires a very high cooling rate which can be achieved by immediately launching the embryos into liquid nitrogen. It has a much higher concentration of cryoprotectants compared to slow freezing. You can see the temperature profile of vitrification in the red line above.
Although these two methods differ, they ensure a higher survival rate and viability of human embryos.
The benefits of a successful vitrification program
In 2011, Rienzi et al1 showed that the contribution of cryopreservation to the cumulative live birth rate in Europe, was around 4%. We can assume that this figure has increased in recent years because of pre-implantation genetic screening and freeze- all cycles.
An efficient cryopreservation program in the clinic is crucial as it provides a lot of benefits including:
- Application of an elective euploid embryo transfer to reduce miscarriage rate
- The opportunity to perform cycle segmentation
- Extends time for embryo evaluation
- Enables egg banking for donation and oocyte accumulation
- Permits fertility preservation
- Enhances the cumulative live birth rate per oocyte retrieval cycle
Vitrification
Vitrification allows us to cryopreserve cells by bringing them into a glass-like structure without any ice crystal formation. An ice crystal formation can be detrimental for embryo viability.

A highly efficient vitrification program should always be maintained in order to achieve higher rates of success.
According to many publications, vitrification is more effective than standard slow freezing when it comes to most stages of embryo development. It can double pregnancy rates.
Because of the high efficiency of vitrification, there are a lot of commercial kits and vitrification devices available which vary between different laboratories.
Vitrification cooling protocols
Commercial vitrification kits are similar in composition and protocols even though they may be different.
Vitrification is a two-step procedure which requires moving samples from the drop with equilibration media to another one with vitrification media. Here are two examples of popular protocols used with success for embryos.
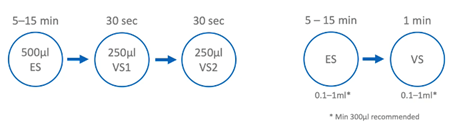
The main differences between commercial protocols includes:
- The number of vitrification drops, which varies from one to four
- Recommended volume. For example, the SAGE vitrification kit offers flexibility to use the volume from 0.1 to 1ml, while others are restricted to recommendations of 20, 50 or 300 microliter drops
Small volumes work in some hands, but safety and consistency grow with larger volumes. A minimum of 300 microliters is recommended because it has proven successful in popular commercial kits.
Ensure your results are satisfactory and consistent with 300 microliters drop of equilibration solution before attempting to reduce volume. Then you can evaluate the effect.
The total duration of exposure to the vitrification solutions and loading of the vitrification carrier, must not exceed the maximum time stated in the instruction for use (IFU).
Why volume is important
It has been mentioned that volumes of equilibration and vitrification media are crucial for the vitrification process.
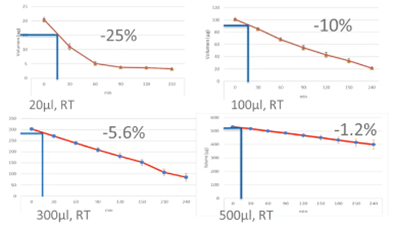
Below is our internal data regarding the weight loss during the equilibration step with different volumes of the solutions.
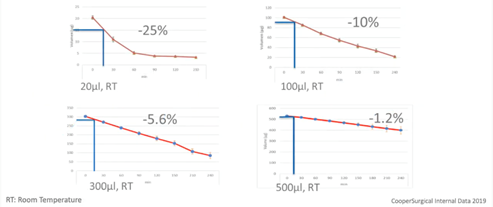
The blue lines represent a 15-minute equilibration of a blastocyst. As you can see, drops size will affect the evaporation and consequently, consistency of the environment surrounding the gamete on embryo.
Ideally, each cryo case should be treated the same way in terms of time, volume, temperature, media etc. This way, variation between cases and operators can be minimized and consistency in outcome will be increased. Large volumes will also make mistakes less likely to affect the outcome.
Commercial kits are similar in composition. The four most used cryoprotectants include dimethyl sulfoxide (DMSO), 1,2- propanediol, glycerol and ethylene glycol. They appear in different variations for different purposes. For example, glycerol is mostly used in kits for sperm freezing while propanediol in combination with dimethyl sulfoxide or ethylene glycol, are the main components for vitrification.
All of them do the same job – they drive dehydration and rehydration processes in the cells. Despite differences in pH buffers, supplements, and slight differences in terms of protocols, all kits have happy users and can be optimized for individual laboratory settings.
There is still no clear difference in favour of one or another when it comes to cryoprotectants. I just want to stress the point that all cryoprotectants can be toxic if used incorrectly.
Vitrification warming protocol
The warming step is one of the most important in the vitrification program. Rapid warming is crucial to avoid ice crystallization which can be detrimental to the survival of embryos.
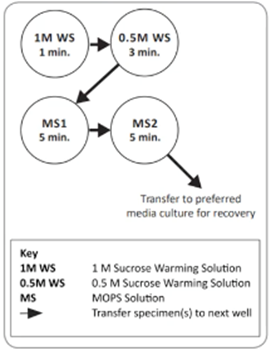
The warming rate is determined by two parameters – volume and temperature of the initial 1M Warming Solution (WS). The recommended volume for this step differs for different commercial kits and protocols but is usually between 0.5 and 4 millilitres.
During this step, intracellular Cryo Protective Agents (CPAs) are rapidly removed from the cells and diluted to prevent their cytotoxic effects. This first step is very similar across different commercial kits, but the next steps can be performed either at room temperature or at 37 degrees, depending on the manufacturer. Just to give you a quick example, the SAGE warming kit is designed to perform all steps at 37 degrees which can help simplify the handling of the procedure.
Universal vitrification/warming protocol
Nowadays, millions of embryos are vitrified using different commercial kits. There are a lot of oocytes, embryos and tissue which have been frozen using the slow freezing technique which are still stored in cryobanks around the world. Furthermore, many clinics still receive samples from other centers that were cryopreserved with different kits and protocols.
In 2014, Parmegiani et al2 published a pilot study with encouraging results, of a single universal warming protocol. It was based on subsequent steps with 1 and 0.5 molar concentrations of extracellular cryoprotectant, irrespective of the freezing protocol, of human oocytes.
The use of a universal warming protocol can simplify laboratory protocols, staff training and management of warming procedures.
In 2018, the same group published a study3 which assessed the clinical efficacy and efficiency of the universal warming protocol on the vitrified embryos. They did this by analyzing cryo survival and implantation rates.
The study was performed in two parts. The first was a prospective randomized control study and it was performed on 350 embryos at the blastocyst stage.
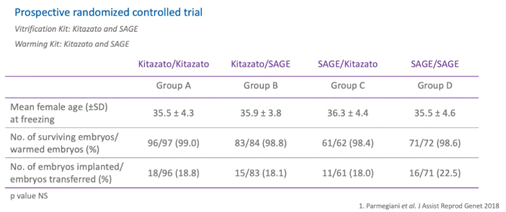
Each embryo was first randomized for vitrification using two different kits – SAGE and Kitazato. Then, at warming, the embryos were randomly allocated to either the SAGE or the Kitazato warming kit.
The universal vitrification and warming procedures were performed for both commercial kits with slight changes. As you can see, female mean age and survival rate were statistically comparable between the study groups.
The blastocyst survival rate ranges from 98.4% to 99%.
Implantation rate in different groups were comparable as well and ranged from 18% to 22.5%.
The second part of the study was a retrospective observational study. It was performed on 1,055 embryos vitrified at the cleavage stage and were obtained from the patient’s own eggs or from egg donation.
All the embryos were vitrified and warmed in various combinations between three different kits – Kitazato, SAGE and an in-house kit. Here, you can see, the results of the retrospective longitudinal cohort study with egg donation derived embryos.
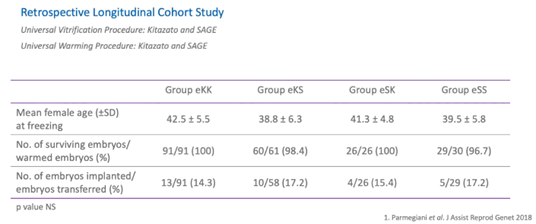
All parameters in study groups like mean female age, embryo survival, and implantation rates were comparable. All the studies support the safe and efficient use of the universal warming protocol.
Now we’ll discuss expectations, the education process and KPIs for the vitrification process.
What to vitrify and what to expect
When implementing a new technique, we usually expect high outcomes. To get consistently good results however, we should be precise and follow very clear established rules.
- Firstly, use all the good quality embryos
- Then, a Standard Operating Procedure (SOP) for verification should be implemented in the clinic to ensure that all small details are taken into consideration
- All operators, regardless of experience, should follow the SOP
- Finally, a learning curve should be implemented
When you implement a new technique in the lab, what results can you expect and how can you manage these expectations?
First, our expectations should be balanced, and the result should be comparable with the fresh data. You cannot expect a 70% clinical pregnancy rate with vitrification cycles if the clinical pregnancy rate from fresh cycles is below 20%.
Top labs are consistent and use large volumes of media.
You can achieve benchmarks only when you optimize quality, experience, and consistency. It’s also important to be realistic. For example, according to the Alpha consensus meeting on cryopreservation key performance indicators and benchmarks4, the implantation rate of human vitrified oocytes may be 10 to 30% lower than in fresh cycles.
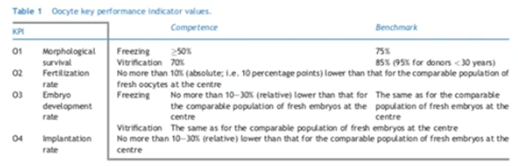
Oocyte cryopreservation: KPIs
What can we expect regarding oocyte cryo-preservation? According to the Alpha consensus, predicted values for survival, fertilization, embryo development and implantation rates, favour the vitrification technique over the freezing method. The implantation rate should be the same as for the comparable population of fresh embryos in the clinic. As we already mentioned however, it could be lower.
KPIs could be affected by the case load and by operator experience.
Cobo et al5 observed differential survival rates among either donors or different stimulation cycles from the same donor after analyzing 40,000 vitrified oocytes in an egg donation program in 2015. 1.4% of cases had a 0% survival rate.
Embryo and blastocyst cryopreservation KPIs
The Alpha consensus came up with KPIs for embryo and blastocyst stage of development as well. The expected morphological survival rate to achieve competence and benchmarks is much higher in vitrification compared to slow freezing techniques.
Overall, for the cleavage stage, it was the consensus that the benchmark for the rate of embryo development should be the same as for the comparable population of fresh embryos at the clinic. It was also concluded that implantation rate should be no more than from 10 to 30% relatively lower than the comparable population of fresh embryos at the center.
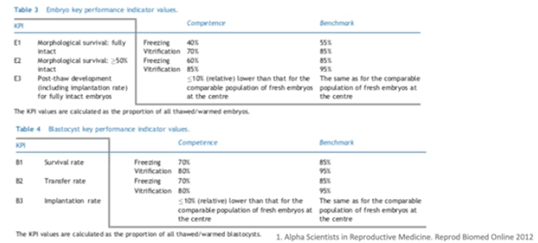
As for the blastocyst stage, survival can be defined as at least 75% of cells perceived to be intact after thawing and warming.
The proportion of thawed/warmed blastocysts that re-expand within two hours, was recommended as an extra KPI to evaluate the survival rate.
Overall, for the blastocyst stage, it was the consensus that the benchmark of the rate of embryo development should be the same as the comparable population of fresh embryos at the clinic. Furthermore, the implantation rate should be no more than 10% relatively lower than the comparable population of fresh embryos at the center.
Consistency
To achieve the competence level and to be closer, or even exceed the benchmark level in vitrification, you should be consistent in all steps of your procedure and carefully manage the main parameters which can affect your results.
Temperature will affect the speed of the process. Normal distribution of equilibration time will be faster at 28-30 degrees Celsius in hot labs, then 20-22 degrees in cold labs.
Usually, protocols are adjusted to room temperature or to 37 degrees Celsius.
As we already discussed, volume is very important. Larger volume may reduce the effect of error. Remember, when dealing with small volumes, you increase the risk of evaporation and increase osmolality in the solution.
Larger volumes are preferable when learning, until satisfactory results are obtained.
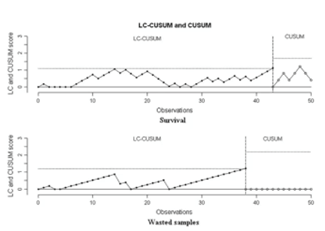
Learning curve
When we implement a new technique in the lab or train a new person, we need an in-house quality control procedure to assess the learning process of the trainee, as well as to monitor the optimized introduction of the new technique.
There are various methods which can be used to determine whether the trainee has reached proficiency. For example, confirming a recommended number of procedures under supervision. A statistical tool designed to indicate when a process has reached a predefined level of performance can be used as well.
According to Dessolle et al6, vitrification procedures appear to be easy to learn even when the trainee is a beginner.
The learning process was mostly limited by manipulation problems. Thus, the learning curve of vitrification for skilled biologists is likely to be shorter, and the introduction of this technology in laboratories should be easier.
Despite this encouraging publication, continuous monitoring after proficiency is required to prevent a performance shift.
KPIs will help you to monitor ongoing performance.
Traceability and witnessing during the vitrification and warming process
As with every step of the IVF procedures, correct identification of gametes and embryos during cryopreservation is very important.
Proper labelling of the cryodevice with patient details such as full name, date of freeze and unique identification number is crucial for the traceability of the sample. Additional information due to the specific needs of the clinic or national guidelines are also applicable.
Witnessing of each step of the procedure from gamete retrieval to the placement of the sample in the cryo cane, ensures the safety of both yourself and the patient during treatment cycles.
Special attention should be paid to documentation of samples in storage for easy identification, especially when samples reach the legal storage limit.
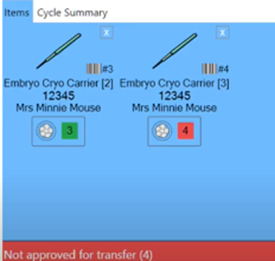
Of course, it’s also very important to identify the individual frozen embryo, especially for clinics utilizing genetic testing. The labels or barcodes hold the identity of individual embryos. Below is an example of a barcode which includes all the necessary information.

There are different types of labels to suit various carriers, like straws or vials.
Witness controls which embryos can transfer
Because every cryopreserved embryo has its individual barcode, you can easily designate the embryo according to ‘approved’ or ‘not approved’ for transfer. This is based on the genetic testing results.
Of course, witnesses cannot automatically approve or reject the embryos based on results coming back from genetic labs. It will still be the responsibility of the lab to evaluate the genetics results and approve the embryos accordingly.
If anyone attempts to thaw or transfer an embryo which hasn’t been approved, the system will provide an audio and visual alarm.
Tips and trick to improve results
First, I’ll share some tips regarding post-biopsy blastocyst vitrification as it’s one of the hot topics nowadays.
There’s still no clear evidence in terms of an optimal time for vitrification after trophectoderm biopsy. Common practice suggests that vitrification should be done within 30 minutes, but more data is needed.
It’s important to start the vitrification process only after cell tubing is completed.
It was shown that trophectoderm biopsy by itself doesn’t impair blastocyst post-warming survival and live birth rates.
Finally, apply the same vitrification practice as standard, as per what has been vitrified in the IVF laboratory.
If the blastocyst has collapsed but you don’t collapse for vitrification, allow it to re-expand.
If the blastocyst has collapsed and you collapse prior to vitrification, vitrify before the embryo area re-expands.
How to start vitrification successfully
There are many factors which should be taken into consideration to start successfully or to improve vitrification outcomes in your clinic.
- First, only good quality gametes and embryos should be selected for cryopreservation. You can still vitrify embryos with questionable quality, but don’t expect the same outcomes you would get when using good quality embryos
- Be very careful with control of timings, temperature, osmolality, and pipetting
- Evaluate time for equilibration as it may differ between labs and stages of embryo development. For example, it usually takes less time for day three embryos to equilibrate compared to expanded blastocysts
- Load the embryos in minimal volumes to support an appropriate cooling rate
- Be very careful with handling, transporting, and storing devices
- Remember that the warming step is crucial and reveals the sum of all prior steps of which each may be detrimental if mistakes occur
Warming regarded most critical step
Most current efforts are aimed at achieving a very high cooling rate using open pulled straws.
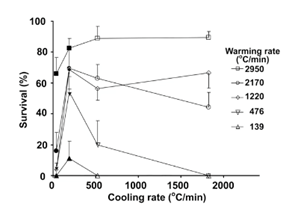
Publications often include some discussions of the possible contribution of the warming rate. There were only a couple of experimental attempts to separate the relative importance of each step. Seki et al7 used the mouse model to show the importance of the warming rate.
In the experiment, the faster the warming rate, the better the survival rate.
Here are some tips to maintain appropriate conditions for the warming step.
- Always keep the warming media at 37 degrees Celsius and use a minimum of 0.5ml volume
- It’s better to pre-warm the media and dish in advance
- Place the carrier from the liquid nitrogen into the warming solution as fast as possible
Summary
- Vitrification is a highly efficient technique for all stages of human embryo pre-implantation development from oocytes to blastocysts
- Oocyte and embryo quality play a crucial role in the survival rate and outcomes for vitrification protocols
- A universal warming procedure proposed by Parmegiani et al3 can be followed regardless of the vitrification media
- The warming step is as crucial as the vitrification step. Warming rate, temperature and volume can affect the results
- Traceability and witnessing are essential and secure the success of the cryopreservation program
- Initial training and ongoing competency evaluation are essential, with objective KPIs
- As with any technique, the cryopreservation protocol needs to be standardized for each individual lab
About Olga Razina MSc
Olga Razina is an Embryology Advisor at CooperSurgical.
Olga has a keen interest in sperm selection, vitrification of oocytes, embryos and blastocysts as well as trophectoderm biopsy for PGT.
In 2019 Olga joined CooperSurgical Fertility Companies as the Embryology advisor for Russia, the Nordic and the Baltic areas.
Read more about Olga Razina MSc.
Additional References
The following are mentioned throughout the text above and/or are particularly relevant to it. To find out more about each item, please click the link.
- Reference:
- 1 Rienzi et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance, Updated 2017
- 2 Parmegiani et al. Rapid warming increases survival of slow-frozen sibling oocytes: a step towards a single warming procedure irrespective of the freezing protocol?, 2014
- 3 Parmegiani et al. Universal warming protocol” for vitrified human embryos: a retrospective study on combination of 3 different vitrification/warming kits, 2018
- 4 Alpha Scientists In Reproductive Medicine. The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting, 2012
- 5 Cobo et al. Oocyte vitrification as an efficient option for elective fertility preservation, 2015
- 6 Dessolle et al. Slow freezing and vitrification of human mature and immature oocytes, 2009
- 7 Seki et al. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure, 2009
- Procedures:
- ART Products:
- ART Products:

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
