RI Witness Laboratory Integration
Analyse your workflow
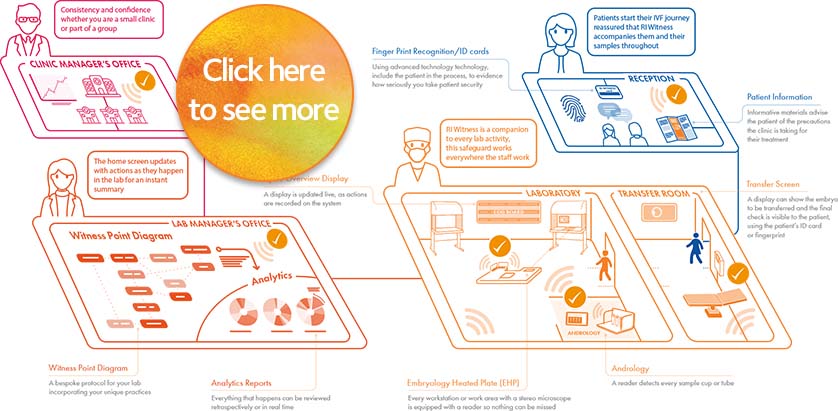
How RI Witness Integrates Into Your Clinic
Constantly communicating with your patient management system RI Witness, either visibly or invisibly, will have an impact at every level. Developed in response to industry voices, there are many features to choose from so you can select as many or as few as you require, leaving the option to expand at a later date. Each installation is tailored to your laboratory, and our installation team will manage your installation from start to finish.
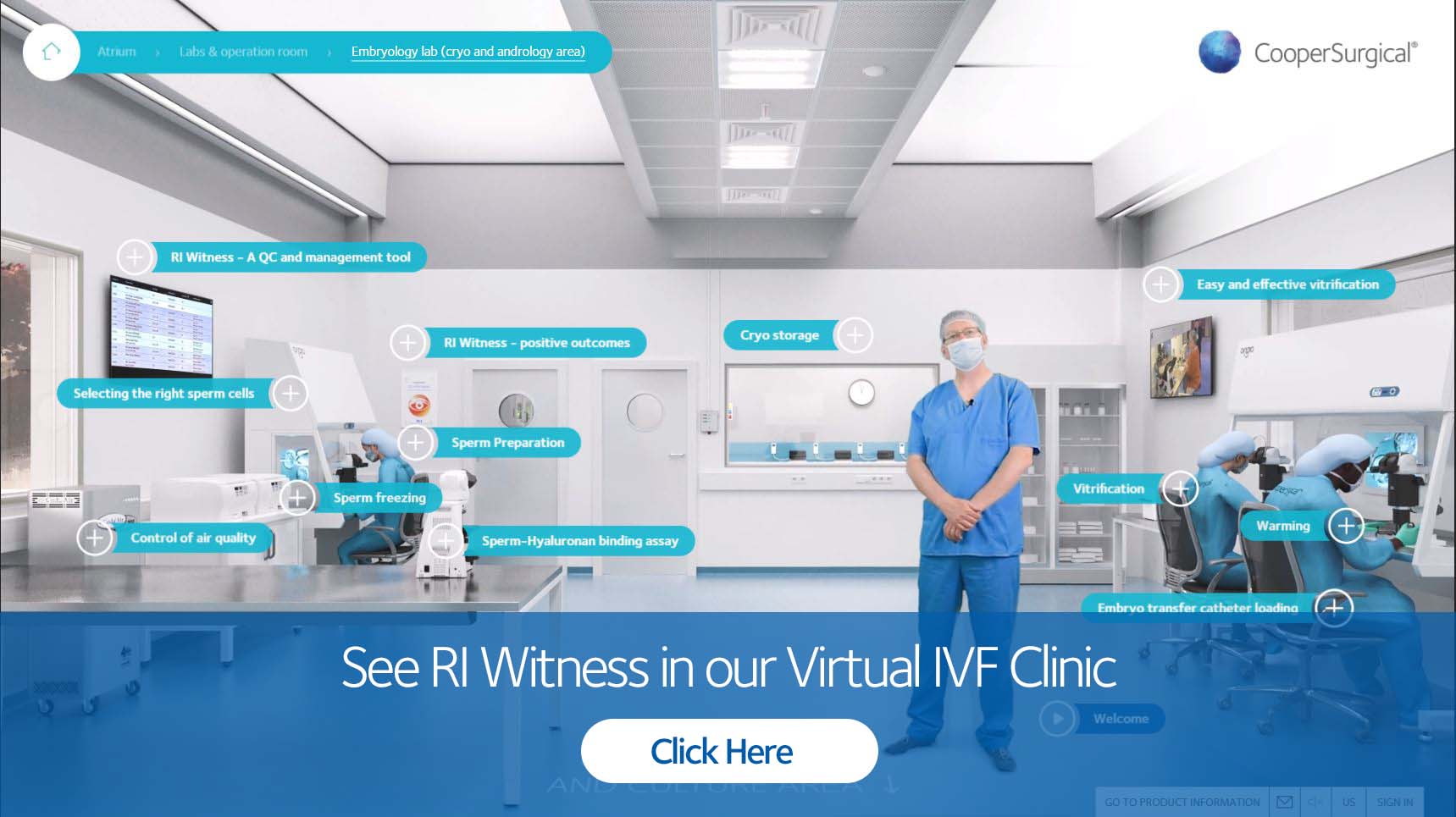
RI Witness brings comfort to patients by constantly observing gametes/embryos at every process step of the IVF cycle, facilitating localization, and identification of all samples.
Integration
We can fit embryology heated plates into all workstations from major suppliers; flush fitted integration is possible with K-Systems and ORIGIO cabinets.
Work areas can be connected through Wi-Fi or LAN cables to a central server which means everything works together and data updates in real time. Our software works with leading patient management databases. We also work closely with clinics and their database administrators on an individual basis to ensure smooth integration with RI Witness.
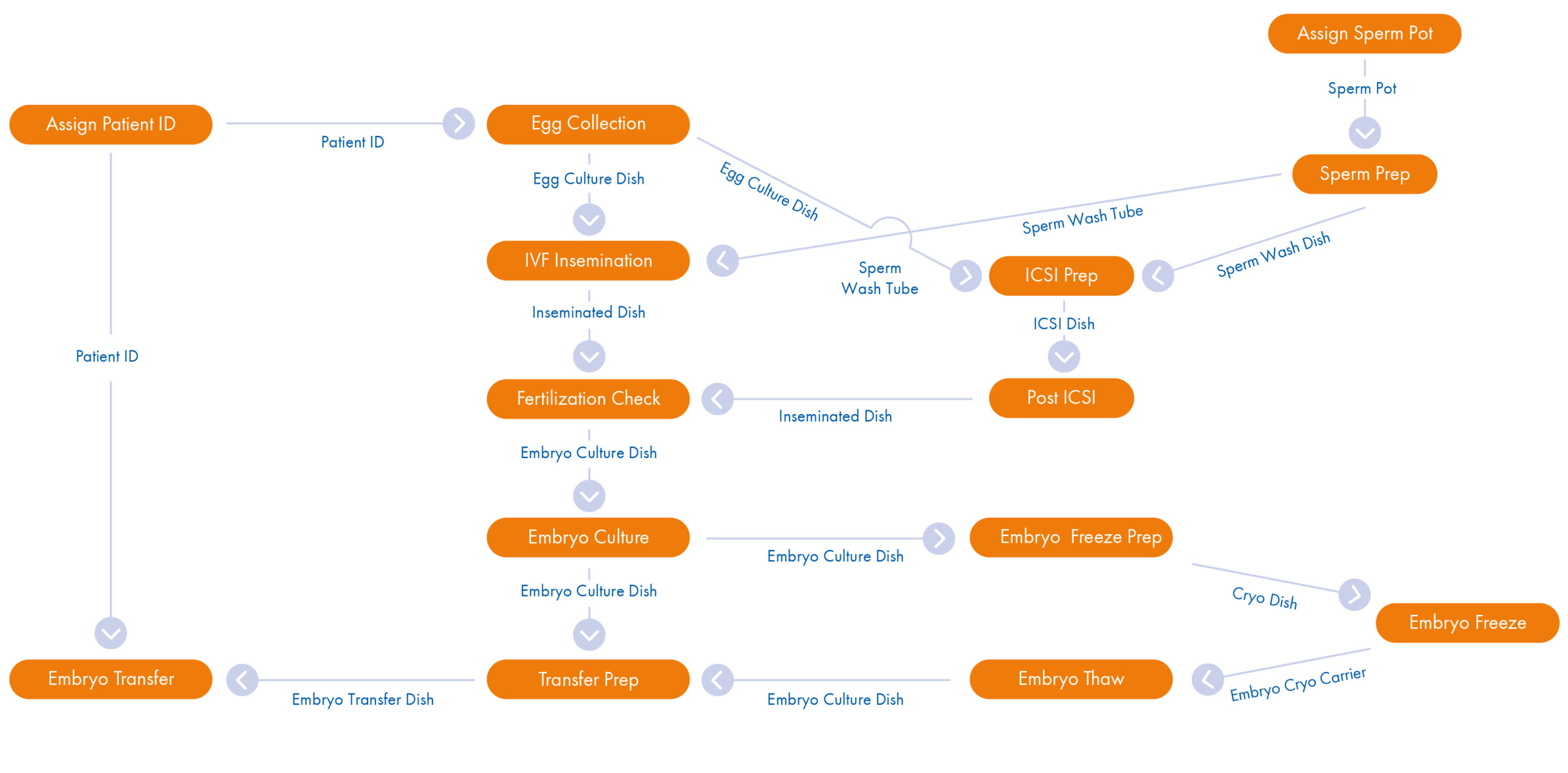
Your RI Witness Point Diagram
Mirroring your existing SOPs the Witness Point Diagram (WPD), created by your clinic’s laboratory management and our installation specialist, provides you with a comprehensive workflow overview. Each critical step carried out during every type of patient cycle is identified and captured in the WPD. Once tested and in place, the WPD allows the RI Witness system to record automatically real-time ‘who, what, where, when and how long’ data across all laboratory activity.
The WPD is flexible and future proof as you will be trained to be able to amend and evolve your protocols ongoing. Different types of cycles can be accommodated.
Implementation and Support
 The logistics and practicalities of implementing a new system of any kind, can be extremely daunting for a busy IVF Clinic. However, our team of specialist installers will do everything possible to ensure a smooth transition.
The logistics and practicalities of implementing a new system of any kind, can be extremely daunting for a busy IVF Clinic. However, our team of specialist installers will do everything possible to ensure a smooth transition.
They will work with you to communicate with clinic database providers to facilitate integration of data, if feasible.
Our installation team will support and guide the Senior Embryologists during the configuration of the workflow ensuring it is precisely how you want it. The nature of the RI Witness system is such that it forces very little change in daily tasks, which means that most normal users will see little difference in how they work. However, the team will ensure users understand the principles of the system and are trained to confidently use it.
Efficiency In The Lab
How can RI Witness make my lab more efficient?
Human double witnessing means a colleague must double check the identity of patient samples at critical points. This has been mandatory in the UK since 2004.
Since then, extensive comparisons have been undertaken to compare human, barcode and the RI Witness RFID systems. The evidence concluded that RI Witness was faster, more efficient, and ensured samples were out of the incubator for less time overall4 when compared to human double witnessing.
Product Specifications
| Work Areas | One work area required for each critical working location. Microsoft Windows based PC or Tablet needed for each work area. Readers available heated or unheated. RFID reader frequency: 13.56MHz |
| Barcode Compatibility (Traceability) | Compatible with GS1 barcodes (GS1-128) |
| Barcode Scanner (Traceability) | Compatible with USB (Keyboard wedge) fixed and hand held scanners |
| Camera Compatibility (Imaging) | Research Instruments’ DC1 & DC2, Analogue cameras |
| RI Witness™ Manager (Client Software) PC System Requirements | Operating Systems: Windows 11, Windows 10 |
| Server / Network Requirements | Microsoft SQL Server required (not supplied). Network Point required for each work area |
Order Codes
The Order Codes for RI Witness™ will depend on your particular configuration.
Brochures
RI Witness IQ Solution Brochure -German
RI Witness IQ Solution Brochure – French
RI Witness IQ Solution Brochure – Italian
RI Witness IQ Solution Brochure – Spanish
RI Witness IQ Solution Brochure – Turkish
RI Witness Patient Flyer – US
RI Witness Patient Flyer – Turkish
RI Witness Patient Flyer – Portuguese
RI Witness Patient Flyer – Czech
RI Witness Patient Flyer – Greek
RI Witness Patient Flyer – Spanish
RI Witness Patient Flyer – Dutch
RI Witness Patient Flyer – German
RI Witness Patient Flyer – French
RI Witness Patient Flyer – Italian
RI Witness Patient Flyer – Arabic
RI Witness Patient Flyer – Bulgarian
RI Witness Patient Flyer – Danish
Same Sex Couple RI Witness Patient Flyer
RI Witness Patient Flyer – Same Sex Couple Male
RI Witness Patient Flyer – Same Sex Couple Female
RI Witness Patient Flyer – Same Sex Couple Female – Spanish
RI Witness Patient Flyer – Same Sex Couple Female – Czech
RI Witness Patient Flyer – Same Sex Couple Male – Czech
RI Witness Patient Flyer – Same Sex Couple Female – French
RI Witness Patient Flyer – Same Sex Couple Female – Portuguese
RI Witness Patient Flyer – Same Sex Couple Male – Portuguese
RI Witness Patient Flyer Same Sex Couple Male – Bulgarian
RI Witness Patient Flyer Same Sex Couple Female – Bulgarian

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada