In this webinar, Steven Fleming, PhD introduces participants to the safe resumption of clinical lab services in the wake of Covid-19 by implementing Good Laboratory Practice (GLP).
Webinar Transcript: Good Laboratory Practice After Coronavirus
In this webinar, Steven Fleming, PhD introduced participants to the safe resumption of clinical lab services in the wake of Covid-19 by implementing Good Laboratory Practice (GLP).
In many countries around the world fertility clinics have been under lockdown due to the Coronavirus pandemic. But restrictions are now being eased, allowing resumption of clinical services. Therefore, we thought it timely to support clinics facing this new challenge by producing this webinar which is the synthesis of information from professional and governmental bodies for scientific literature and our own professional experience.
Genetic make-up and transmission of SARS-COV-2
Who could have imagined that this tiny virus, just 50 to 200 nanometers in diameter, would wreak such havoc on our lives during the past couple of months?
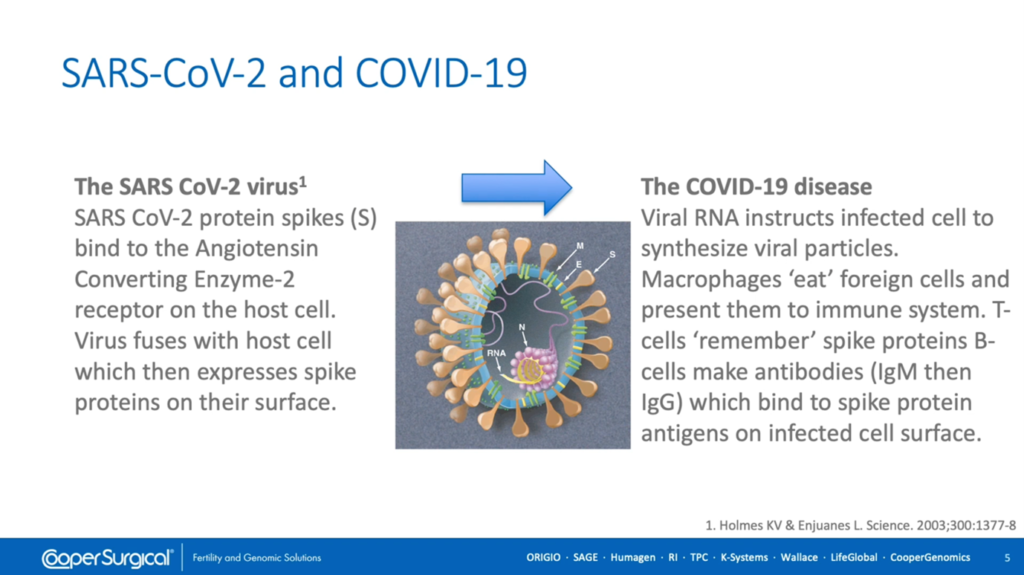
SARS Coronavirus 2 is a strain of severe acute respiratory syndrome related coronaviruses which has close genetic similarity to bat coronaviruses. The RNA encodes four structural proteins; nucleocapsid acid protein (annotated ‘N’ on this diagram) which supports the RNA, and membrane, envelope and spike glycoproteins (annotated ‘M’ ‘E’ and ‘S’ respectively) which, together, comprise the viral lipid envelope which is the virus’ Achilles heel, since lipids are broken down by detergents, alcohol and oxidants such as chlorine.
The virus enters human cells by binding to the receptor, Angiotensin Converting Enzyme-2 (or ‘ACE 2’) by virtue of its great affinity with the extruding spike protein. Infected cells express spike proteins on their surface which are recognized as foreign by macrophages that ingest them and present them to the immune system, including T cells, which remember the spike proteins and B-cells, which make antibodies (initially, IgM and subsequently IgG) that bind to spike protein antigens on the infected cell surface, marking them for destruction by the immune system.
As can be seen in this [above] diagram, upon binding to the ACE-2 receptor, viral RNA is released into the host cell which hijacks its cell processes, instructing it to replicate and disseminate copies of the virus via production of virulence factors which promote budding or shedding of new virions and the viral load peaks three to four days following infection which may be before symptoms appear in the infected individual.
This increased viral load can adversely affect endothelial cells of the airway leading to pneumonia and can inhibit T cells of the immune system. Unlike blood-borne viruses, SARS Coronavirus 2 is primarily spread between people through close contact and is dispersed within respiratory droplets via coughing and sneezing. Indirectly, contact with contaminated surfaces can also lead to infection.
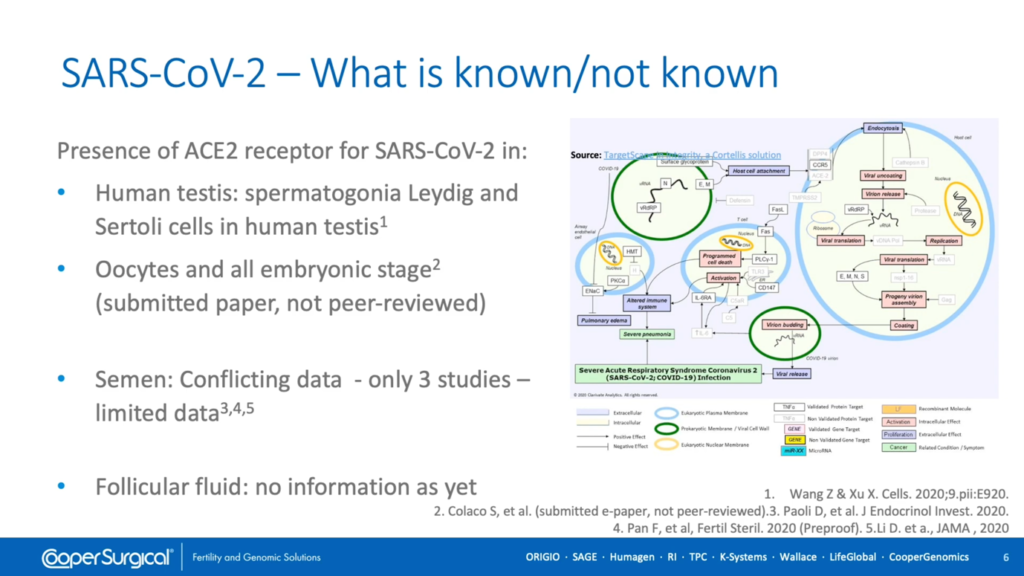
There is limited evidence regarding the presence of the ACE-2 receptor within tissues of the reproductive tract and conflicting data concerning the presence of SARS Coronavirus 2 within semen. So far, there are no reports regarding presence of the virus within follicular fluid.
Planning for good lab practice after Coronavirus
First, it will be necessary to decide how a start-up plan will be drawn up and participation by all team members should be encouraged since they are all impacted by the decisions made. Establish a small working group to coordinate efforts and keep abreast of the rapidly-updated relevant information, including mandatory requirements relevant to your unit.
The need for changes in policy and procedure will be defined by your risk assessments. These will determine what and where changes are required and what changes will mitigate any identified risks. It may also be necessary to consider whether these changes will be temporary (for example, to address the current pandemic) or, indeed, will become the new accepted standard for good laboratory practice.
In determining what changes are necessary, no doubt the plan will identify extra resources – whether people, equipment or consumables such as personal protective equipment – that may require extra funding.
An additional factor that must be considered is that of staff health and welfare. Many people may welcome the chance to return to work but we must recognize that this can also be a stressful time for them. Staff with relatives at higher risk should they contract COVID-19 may be fearful of acting as a vector for the virus and consideration also needs to be given to more vulnerable staff such as those with asthma.
Good laboratory practice when assessing risk of Coronavirus
Risk assessment is central to planning and the areas to consider are likely to include:
- Workflow: how will the work be structured in order to minimize risks?
- By changing work processes it will be necessary to provide additional training, for example in effective use of personal protective equipment (or PPE) and to assess competencies.
- A policy for screening and testing of staff and patients will need to be determined, especially in the event that anyone tests positive.
- Safe, effective cleaning and disinfection routines will need to be decided upon.
- Existing equipment may need revalidation and reassessment of how it is used and further equipment may be required.
- An inventory risk assessment helps ensure that, by start-up, there are sufficient key consumables available such as media, pipettes and catheters and will identify any new requirements such as additional PPE and should include the logistics of deliveries during and after a lockdown.
- Are additional safety measures for cryostorage necessary?
- Monitoring of outcomes will prove critical as it is likely that every laboratory will be changing at least some of their practices.
And finally, what networking will be necessary to ensure you remain in contact and up to date with the wider IVF community so that you can maintain the highest possible standard of care for your patients.
Identifying and managing Coronavirus risks in assisted reproductive technology labs
There’s still much we do not know but identified areas of potential additional risk relate to patient-to-patient, patient-to-staff, staff-to-patient and staff-to-staff exposure to SARS Coronavirus 2. Measures that can be taken to reduce these risks include reducing patient visits by trimming down monitoring and using online consulting and by encouraging off-site sample collection.
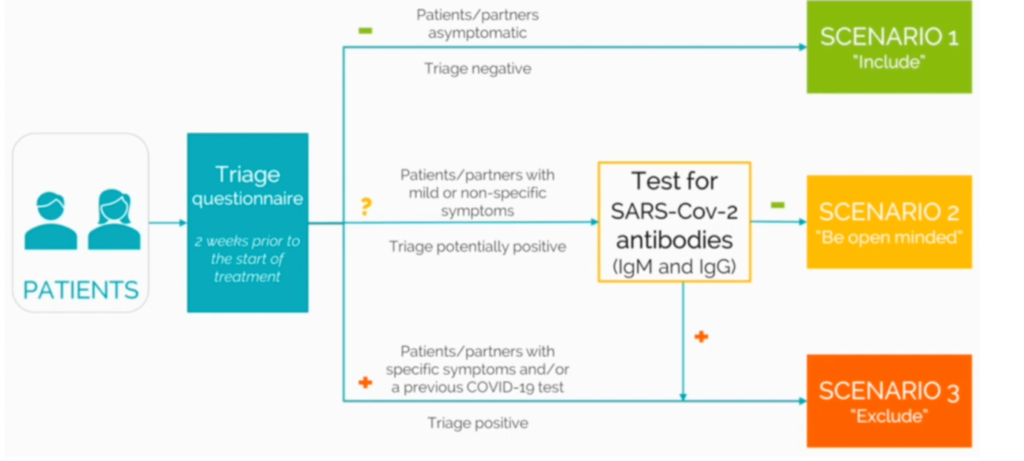
ESHRE have recommended that patients be submitted to a triage questionnaire two weeks prior to the start of their treatment to determine whether testing is required and whether treatment may commence.
Risks to staff relate to their proximity to others and the degree of protection from infection available to them. Limiting the number of staff in the laboratory, dedicating uncluttered work areas for specific tasks and individual use of pens and other handheld devices supports social distancing and will reduce the risk of infection.

The photo on the left illustrates how relaxed we used to be and the photo on the right is the polar opposite approach. The outcome of a risk analysis is likely to fall somewhere between these two extremes with good hygiene and comprehensive use of personal protective equipment (such as lab scrubs, hats, masks and gloves) being central to risk management. Specific procedures involving patient contact may require additional PPE such as eye shields.
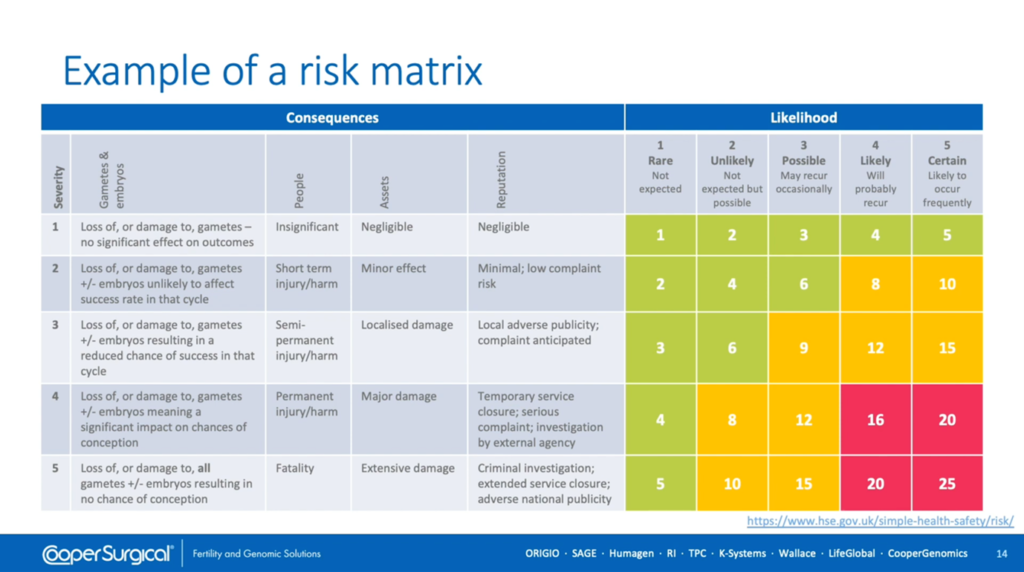
Shown here [above] is a risk matrix which is a classic way in which to evaluate the severity and likelihood of a specific hazard resulting in a consequence and can be used to derive a risk score, colour-coded here as low, medium or high risk. This assesses risks identified in your laboratory and helps you evaluate how to reduce the risk score.
Good laboratory practice when cleaning for Coronavirus
Next we will consider cleaning which is essential for controlling microorganisms and viruses.
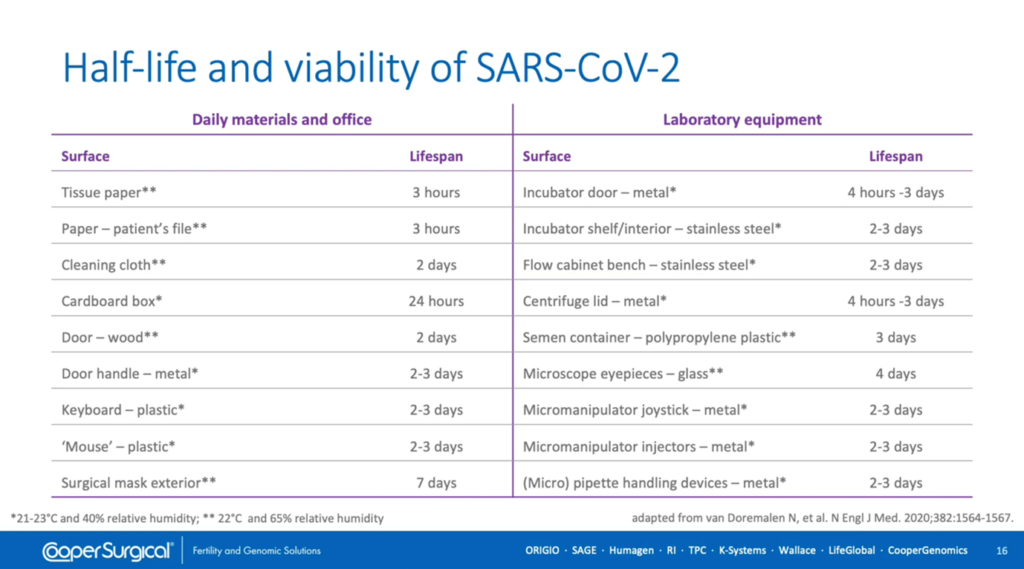
The risk of contamination by SARS Coronavirus 2 has been shown to be dependent upon the half-life of the viral titer on various surfaces and it can be seen from the left-hand side of this table that there’s great variability in how long this virus will persist depending upon where it lands. On paper and cardboard it has a relatively short life-span but on metals and plastics it lasts much longer. However, there’s some debate over how readily these controlled lab experiments translate into real-life exposures. Alarmingly, the longest survival period is on the exterior of surgical masks, presumably because they provide a moist environment, which highlights the need for their regular replacement.
We can extrapolate these findings to lab equipment on the right-hand side of this table where, again, it’s equipment constructed of metal, plastic and glass which is of most concern.
When it comes to choosing a suitable disinfectant, many factors need to be considered including its efficiency at destroying microorganisms and its safety with respect to the cells and tissues being handled, as well as to the staff handling them. It is likely the disinfectants
comprised of volatile organic compounds will be harmful since it is known that these easily cross the lipid cell membrane and thereby can disrupt embryo development. It is also preferable that the disinfectant is non-corrosive and leaves no toxic traces.
A deep-clean needs to be conducted before starting up and should probably be repeated every six months. Additionally, it’s important to disinfect work surfaces at the beginning and end of each day as well as between each patient. The outer packaging of disposables and media should be removed with gloves and their contents cleaned before bringing them into the laboratory. When disinfecting lab fixtures and equipment, it’s worthwhile paying particular attention to hatches and doors to clinical procedure rooms. Such hot spots for microbial contamination can be identified using microbiological tests. It’s also important to log all cleaning activities for traceability.
Validation of laboratory equipment and consumables
In implementing good laboratory practice it’s essential to have good equipment and this can easily be the weakest link. It’s important to establish that the equipment does what it’s meant to, that there’s enough of it and that it works according to specifications so is fit for purpose. It’s also important for staff to know that it’s been set up correctly and be trained to use it.
It’s a prerequisite, as detailed within the ESHRE revised guidelines for good practice, that equipment is appropriately validated as defined by the ISO 15189 standard. In essence, this means that we have to check the all equipment operates within agreed specifications and that there are measurements and data recorded to support this.
Tests can be performed to determine that the equipment is working correctly (termed ‘Operational Qualification’) and then repeated under normal working conditions to show that this is reproducible (termed ‘Performance Qualification’). Note that any devices used to perform the testing must be certified and calibrated. One would expect all key parameters to be tested including carbon dioxide, oxygen, temperature and air quality.
Temperature-mapping should reflect the way in which the equipment is used. Additional tests (for particle counts and volatile organic compounds for example) will require specialized testing devices or external support.
If incubators have been left running, they may simply require airing out before use by regularly opening the incubator doors and chambers. However, if they have been switched off, it will be necessary to purge the gas lines and replace any humidification devices and
In-line gas feed filters. It is also worthwhile considering allocating patients dishes to individual areas of the incubator, making allowances for cleaning between patients.
Work should be allocated to Class I or Class II workstations depending upon its relative risk to staff. Lasers should be checked for calibration and alignment and ancillary equipment may also require temperature-mapping and calibration.
Preparation of the laboratory must also include management of resources in addition to personnel. Access for servicing and repairs needs to be reviewed, along with the availability of spare parts and consumables. Workload, delivery schedules and transport logistics may all impact upon this so careful management is likely to be critical.
Cryopreservation policies and procedures
The potential or perceived risks of cryopreservation include whether the operator and/or cells and tissues being cryopreserved might act as vectors for disease and whether there’s a risk of cross-contamination during cryostorage so it’s important to consider how these risks might be reduced.
If we question whether an embryologist could transmit a pathogen to straws and other devices, then we have to answer ‘Yes’. Furthermore, the operator could transmit a virus to other operators and to the human samples being stored. Therefore, good hygiene is important and it’s worthwhile considering using disposable gloves inside cryogenic gloves to minimize transmission and to avoid sharing protective eye equipment between staff without washing it beforehand. Electronic witnessing will also help maintain safe distances between staff.
As mentioned earlier, there’s limited evidence regarding the presence of the ACE-2 receptor within tissues of the reproductive tract and conflicting data concerning the presence of SARS Coronavirus 2 within semen. And, besides, detection of viral proteins and/or nucleic acid doesn’t necessarily mean that infectious virus particles are present. So we have to assume the possibility exists that these tissues could act as vectors for disease and that embryos with a breached Zona Pellucida may be at greater risk. However, apart from mycoplasma, there have been no reports of viral cross-contamination between oocytes and embryos during cryopreservation.
Over the long term, ice sediment accumulates within storage dewars and poses a contamination risk to stored human samples. Ice forms within liquid nitrogen via two general processes:
- It forms in the atmosphere above an open dewar and falls into the vessel, or
- It forms upon cold surfaces of the dewar during inventory of stored material.
Ice crystals aggregate and entrap other materials such as bacteria, fungal spores and general lab debris present within the liquid nitrogen. Since contamination of human samples, liquid nitrogen and cryogenic tanks by microorganisms and viruses remains theoretically possible, high-security straws and closed vitrification devices may be preferable.
According to your lab procedures, straws should be disinfected before warming, taking care not to harm the human material contained within them. Nevertheless, a pragmatic approach needs to be taken in view of the lack of evidence for cross-contamination between human samples.
Further measures that can be taken to minimize the risk of cross-contamination include cleaning and decontamination of storage vessels and adopting sterile technique during cryopreservation. UV or filter sterilization of liquid nitrogen is an option but is labour-intensive and costly.
Theoretically, closed devices avoid exposure of human material to liquid nitrogen but exhibit different cooling and warming rates to open devices. Conflicting biological and clinical outcomes have been reported for oocytes and embryos using open and closed systems. However, a systematic review and several meta-analyses showed no significant difference between open and closed carriers. At warming, it has been shown experimentally that using sterile liquid nitrogen to wash the carrier – and performing several washes of oocytes and embryos – dilutes out the viral titer to negligible levels.
Vapor phase cryostorage may be perceived as less of a risk for a cross-contamination but, compared to liquid nitrogen, it provides a temperature gradient rather than a uniform temperature though the graph demonstrates that it maintains a stable temperature over time.
However, care has to be taken to minimize the duration of opening of the tank so as not to disturb the temperature gradient and there is still a risk of viral transmission via contaminated ice crystals on the tank lid. Continuous alarmed monitoring and backup is mandatory for both liquid and vapor phase storage.
Further information on managing the risks of cryopreservation is provided in national and international guidelines and can be found in our knowledge hub. Good laboratory practice dictates that all samples should be treated as if positive for viruses and therefore the risk of cross-contamination may be mitigated by storing samples testing negative separate from samples that may be positive. The storage of samples testing positive for multiple pathogens requires a further risk assessment, however.
Staff health and welfare
It is to be expected that staff may be anxious regarding their employment and their risk of infection and viral transmission to their relatives on returning to work. To ease anxiety and minimize risk, it is important that all staff receive training regarding the risks of COVID-19 and good safety practices to avoid infection.
Screening and testing options should be considered, taking into account local guidance. All staff should be provided with work clothing and footwear and must have access to personal protective equipment. It is recommended to have in place emergency plans for unintended exposure of staff to infection and to make staff aware of local sources of emotional and psychological support such as those available in Australia.
As with patients, ESHRE recommend using a health and lifestyle questionnaire to triage staff to determine whether they are fit to return to work. In the workplace, the risk of infection to staff can be reduced by limiting their interaction with patients and other staff members by using non-overlapping teams and by having a zero-tolerance policy towards the unnecessary presence of others within the laboratory.
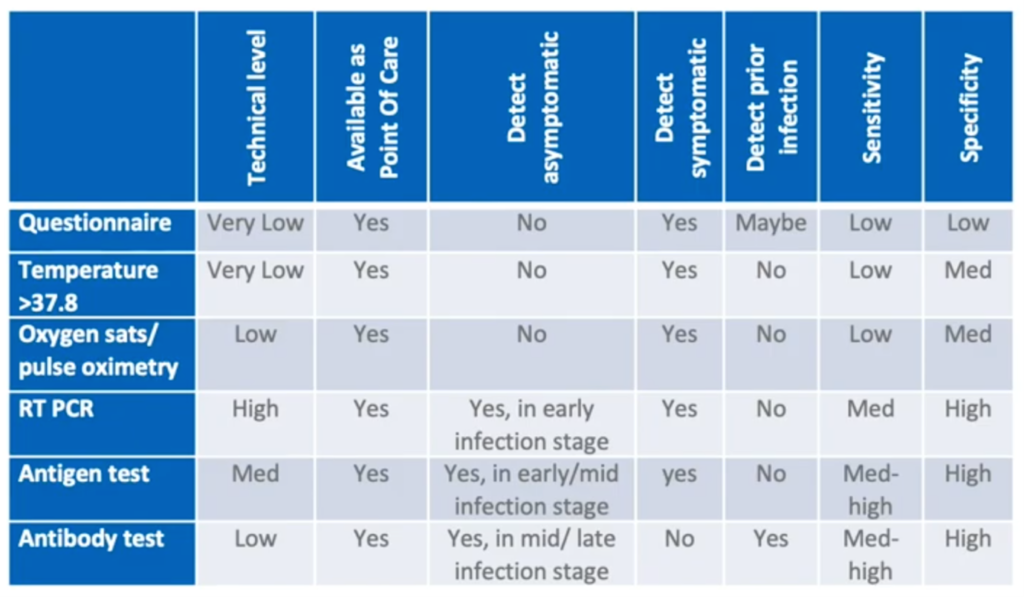
Timely and accurate screening is essential to healthcare management and at-the-door screening should be made available for all staff, patients and visitors. As can be seen in this table [above], there are different approaches to screening and testing. Though the RT-PCR test of the nasopharyngeal swab has high specificity for symptomatic and asymptomatic individuals at an early stage of infection, it also requires a high level of technical expertise to perform. Therefore, using questionnaires and taking temperature offer a more pragmatic approach, especially since screening will need to be repeated at regular intervals.
Use of Class II safety cabinets is recommended for the protection of staff while handling potentially hazardous samples. Whereas Class I laminar flow cabinets direct air towards the operator, Class II safety cabinets draw air away. However, it’s important to avoid placing unnecessary items within the cabinet which might impede normal air flow and it’s still necessary to wear personal protective equipment.
The wearing of personal protective equipment and the need for social distancing are here to stay which presents an impediment to the requirement for double witnessing of patient and sample ID. Electronic witnessing overcomes this problem to a great extent (enabling staff to work alone) and minimizes the number of staff required in the laboratory.
Summary: Steps to take to ensure good laboratory practice after coronavirus
- Plan the lab start-up and appoint a coordinator
- Appoint a risk manager to conduct a comprehensive risk assessment
- Check local guidelines
- Validate all procedures within your own setup
- Perform a deep clean and disinfect the laboratory and equipment
- Validate and calibrate all equipment, especially incubators according to manufacturer’s instructions
- Conduct a full inventory of consumables, especially personal protective equipment
- Update your standard operating procedures and retrain all staff
- Ensure they have access to PPE and infection testing
- And, finally, stay informed and keep up to date with what we know about COVID-19
Useful links
If you would like to explore any of the products / references mentioned in this article, please click on the links listed below:

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
