
Admittedly, in vitro maturation (IVM) experienced a problematic and misunderstood adolescence but, with increased knowledge and understanding of both the clinical and scientific mechanisms involved, it is no longer considered experimental.1 Steven Fleming PhD, our Director of Embryology, reintroduces the IVM approach in this insightful article.
Clinically, IVM was originally considered most suitable for women with polycystic ovaries where it was employed to mitigate the risk of ovarian hyperstimulation syndrome.2 However, the indications for IVM have now been extended to include women having a high antral follicle count3 and in conjunction with oncofertility preservation.4
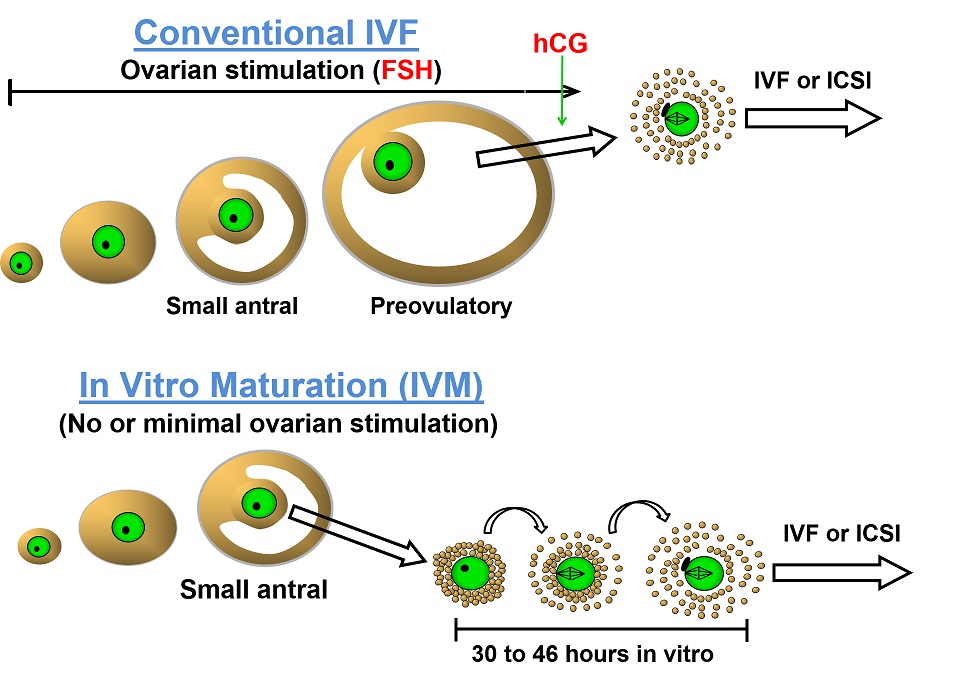
Furthermore, perhaps there is also a case to be made for its use in oocyte donors who do not themselves benefit from controlled ovarian hyperstimulation and may wish to avoid its potential side-effects by using IVM, which requires little or no ovarian stimulation (Figure 1).
Figure 1: Diagrammatic comparison of conventional IVF and IVM5
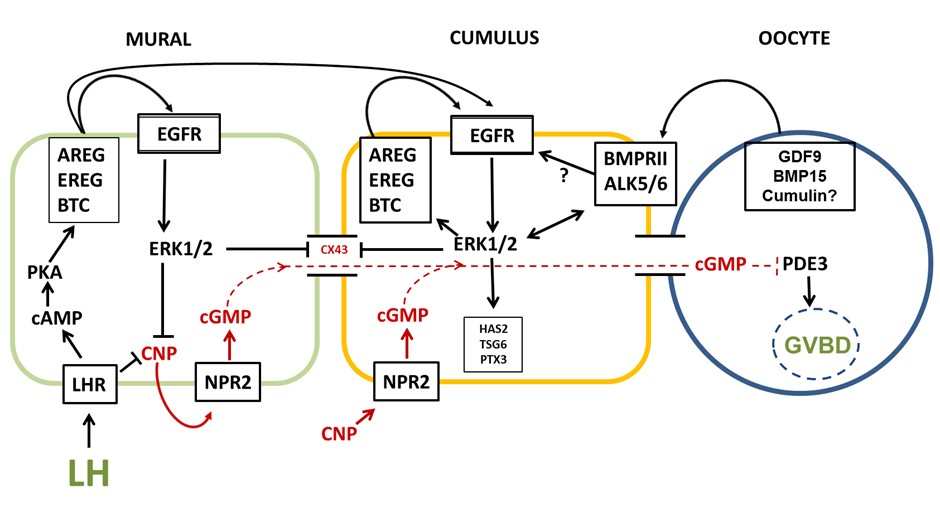
We now know the key regulators of oocyte maturation within the ovarian follicle (Figure 2) and with this knowledge has come a clear understanding of the value of a biphasic in vitro culture system that allows capacitation of the oocyte prior to its maturation6, much like a spermatozoon requires capacitation prior to being able to fertilize an oocyte.
Unsurprisingly, an immature oocyte within a small follicle requires additional time in which to attain further growth and developmental capacity that otherwise would occur in vivo in response to continued ovarian stimulation.
Figure 2: Regulation of meiotic maturation within the ovarian follicle7
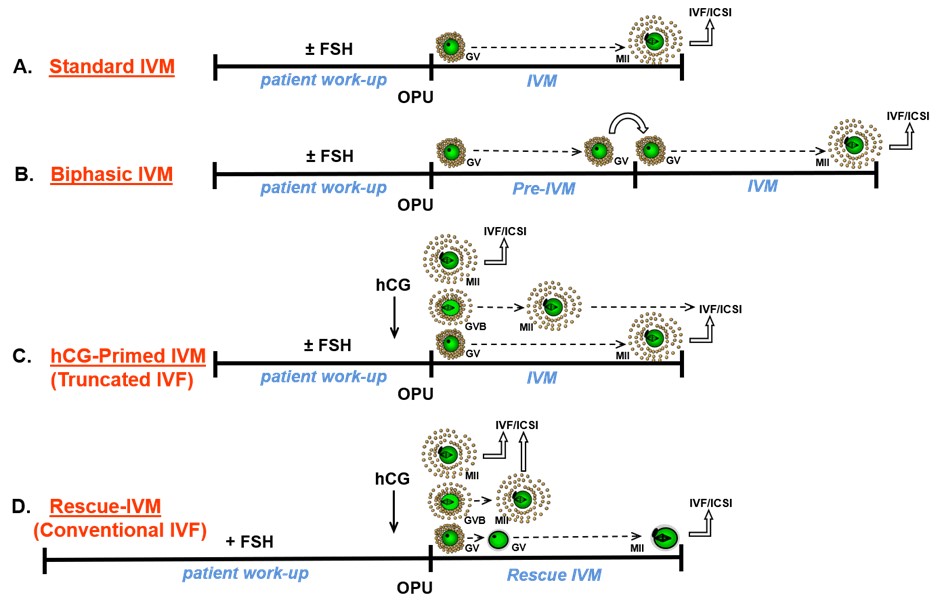
Distinctions between in vitro fertilization (IVF) and IVM have been blurred by the various use in IVM of minimal or no ovarian stimulation and triggering or not triggering resumption of oocyte maturation with the administration of human chorionic gonadotrophin (Figure 3).
Such variations appear to differentially impact endometrial receptivity since fresh embryo transfer following IVM does not seem to achieve as good clinical outcomes as transfer of cryopreserved IVM-derived embryos in a subsequent natural or programmed hormone replacement cycle.8-10
Figure 3: Diagrammatic definition of IVM and variants thereof5
Conclusion
As rates of blastocyst development and live birth with IVM are now approaching those following IVF5, IVM appears to have truly come of age!
 Steven Fleming PhD is an Honorary Associate at the University of Sydney and Director of Embryology for ORIGIO. Previously, he was the founding Scientific Director of Assisted Conception Australia, and a Senior Fellow at the University of Queensland.
Steven Fleming PhD is an Honorary Associate at the University of Sydney and Director of Embryology for ORIGIO. Previously, he was the founding Scientific Director of Assisted Conception Australia, and a Senior Fellow at the University of Queensland.
After completing his doctorate in 1987, he undertook postdoctoral research at the Royal North Shore Hospital in Sydney, and from 1993-1997 he was appointed Lecturer in Obstetrics and Gynaecology at the University of Nottingham in England, where he established the world’s first Master’s degree in Assisted Reproduction Technology with Simon Fishel PhD.
From 1998-2008 Steven was the Scientific Director of Westmead Fertility Centre in Sydney and appointed Senior Lecturer in Obstetrics and Gynecology at the University of Sydney. He is the recipient of numerous research grants, and an author and editor of several books, book chapters and peer reviewed journal articles. Steven’s research interests include cryopreservation, endometrial physiology, endometriosis and oocyte maturation, among others.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada