How endometrial receptivity testing can help patients with recurrent implantation failure (RIF)

How endometrial receptivity testing can help patients with recurrent implantation failure (RIF)
This article will explore the challenges faced by patients with recurrent implantation failure and examine various factors that can impact the success of implantation. We will examine the positive reproductive outcomes in the recent Ohara et al. (2022) study using CooperSurgical’s ERPeak and discuss CooperSurgical’s new 3-in-1 endometrial receptivity test, ER-CompleteSM.
Recurrent implantation failure is a challenging and frustrating condition for both patients and clinicians
In vitro fertilization (IVF) advancements have opened the opportunity for successful fertility outcomes for many patients and equipped clinics with newer and bolder technology.
Unfortunately, a population of patients exist that struggle with RIF which is characterized by three or more unsuccessful IVF cycles utilizing good-quality embryos.1 For such patients and their clinicians, this is a frustrating situation since there may be more than one pathological cause.2
“In addition to the emotional and financial stresses of implantation failure, the difficulty in determining the exact cause of RIF can cause patients additional ongoing stress,” according to Tony Gordon, Senior Director Clinical Strategy and Market Development at CooperSurgical Inc. “This uncertainty can also create issues for both patients and clinicians in determining the most appropriate course of treatment.”
The significance of embryo-endometrial synchronization and optimal microbiome in implantation
We’ll discuss some considerations here that can help identify an ideal endometrial environment and optimal time for embryo transfer: an accurate window of implantation (WOI), a favorable endometrial microbiome devoid of detrimental pathogens or bacteria and a robust accuracy in endometrial receptivity testing.
“Endometrial receptivity testing that identifies a displaced WOI can help guide clinicians to move the day of implantation either to a day or a day earlier to optimize the chances of sustaining implantation.”

Tony Gordon
Senior Director Clinical Strategy and Market Development, CooperSurgical Inc.
Window of Implantation
For the majority of RIF patients, the WOI is five days after progesterone administration in an IVF cycle. However, for approximately one-third of RIF patients, their WOI is displaced beyond day five to day six, meaning an embryo transfer (ET) at day five is too soon and their endometrium will not be in an optimally receptive state and less likely to support implantation.3
Conversely, about one-tenth of RIF patients have a day four WOI rather than the expected day five, therefore, a day five ET is too late and past their optimal receptivity, reducing the chance of implantation.3
In several studies where a substantial portion of RIF patients suffered from a displaced WOI, statistically significant results were found with personalized embryo transfer (pET) compared to non-personalized embryo transfer (npET) without endometrial receptivity (ER) testing.3, 4
“ER testing that identifies a displaced WOI can help guide clinicians to move the day of implantation either to a day later (pre-receptive) or a day earlier (post-receptive) to optimize the chances of sustaining implantation,” says Dr. Gordon.
Endometrial Microbiome
Studies have shown that a healthy endometrial microbiome, characterized by a high abundance of lactobacilli, may be associated with successful implantation.5, 6 Additionally, higher levels of lactobacilli may indicate the absence of endometritis, a chronic inflammation of the endometrium which is a known contributor to implantation failure.7 It is worth noting that chronic endometritis can be present without any symptoms, and can be found in up to 40% of RIF patients.5
An endometrial sample is taken from a minimally invasive biopsy. Biopsy testing allows clinicians to discover the histology and microbiological status of the patient’s endometrium, enabling them to perform a pET at the optimal time and helping improve the chances of successful implantation.7, 8
To improve IVF outcomes, endometrial receptivity testing should offer accurate results of proven clinical benefit. A recent study demonstrated significant benefits for CooperSurgical endometrial receptivity testing in RIF patients.
“…this study [strengths] include its large sample size (1000 patients in total), its focus on RIF patients, and the fact that >99% of patients required a single biopsy which reduces the need for rebiopsy.”
New large cohort study on RIF patients explores CooperSurgical’s ERPeak® test
A retrospective cohort study by Ohara et al. (2022) evaluated the clinical outcomes of RIF patients in two age groups: advanced maternal age (AMA) and non-AMA, comparing non-personalized embryo transfer and personalized embryo transfer based on ERPeak testing, which uses real-time quantitative polymerase chain reaction (RT-qPCR). This method is reported to have the most sensitivity and least bias when analyzing gene expression.9 Forty-eight genes found to explain >99.5% of total sample variance were analyzed.10
Some strengths of this study include its large sample size (1000 patients in total), its focus on RIF patients, and the fact that >99% of patients required a single biopsy which reduces the need for rebiopsy.
In the experiment group, clinicians utilized pET guided by ERPeak testing on 480 RIF patients. The results found 271 (55.4%) patients were found receptive, and 209 patients (44.6%) had a displaced WOI; within the latter, 62.2% were determined to have pre-receptive WOIs and 37.8% were determined to have post-receptive WOIs.3
When observing AMA and non-AMA in the 480 RIF patients, there was no significant difference in the frequency of displaced WOI detected between the two groups, however, the rate of pre-receptive status was higher in AMA patients compared to non-AMA.3
ERPeak significantly improves clinical pregnancy and live birth rates
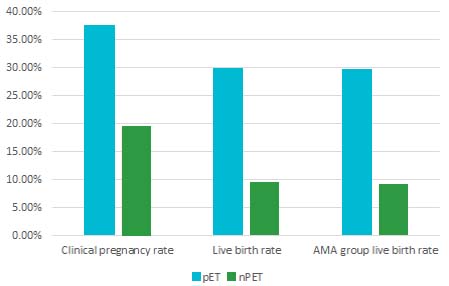
The Ohara study observed significantly higher reproductive outcomes per embryo transfer for pET with ERPeak: the clinical pregnancy rates doubled, birth rates tripled, and miscarriage rates halved for RIF patients compared to npET.3
Outcome comparisons: pET with ERPeak vs. npET
The primary objective of ER testing is to increase pregnancy and live birth rates, but the Ohara study revealed an unexpected benefit: a significant reduction in miscarriage rates among AMA patients, which was even more pronounced in non-AMA patients.3
Miscarriage rate
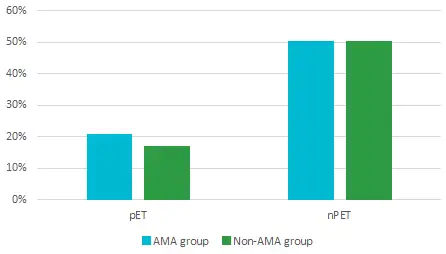
For Dr. Gordon, “these findings suggest that transferring embryos at the optimal window of implantation not only improves implantation rates but also reduces the risk of miscarriage. Overall, ERPeak should be seen as a viable option for AMA and non-AMA RIF patients looking to improve their chances for a successful reproductive outcome.”
New 3-in-1 comprehensive endometrial receptivity test
CooperSurgical’s ER-CompleteSM endometrial receptivity test combines the ERPeakÒ and ERBiomeSM tests to help determine the optimal time and environment for embryo transfer.
The ERBiome test uncovers insights into the endometrial microbiome including lactobacillus levels present and the detection of detrimental endometrial reproductive pathogens.
Endometrial dysbiosis, an imbalance of microbes can result in a condition called endometritis which has been shown to negatively impact embryo implantation and pregnancy outcomes.11, 12 The ERBiome test detects specific reproductive tract pathogens associated with dysbiosis and endometrial inflammation, both of which can contribute to infertility.13
“Next-generation sequencing technology can be used to identify reproductive tract pathogens without the need for culturing, and potentially detecting more microorganisms,” states Dr. Gordon.
The ERBiome test also evaluates the lactobacillus levels present in an endometrial biopsy sample. Patient endometrial samples are classified as lactobacillus dominant (LD), with an abundance over 90%, or non-Lactobacillus dominant (NLD). An endometrial environment high in lactobacillus has been shown to be conducive to improved reproductive success, including implantation, pregnancy, ongoing pregnancy, and live birth rates.6
As Dr. Gordon explains, “By combining the detection of reproductive tract pathogens and lactobacillus levels via ERBiome with the determination of the window of implantation via ERPeak, we can provide patients with comprehensive information from a single biopsy. Patients and clinicians can then discuss ER-Complete results and the various options available for either altering day of implantation and/or treating lactobacillus levels or the presence of reproductive pathogens, with the goal of increasing the chance of sustained implantation.”
Relevant articles
Relevant knowledge
Relevant products
References
- Bashiri A, Halper KI, Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):121.
- Franasiak JM, Scott RT. Embryo and Endometrial Synchrony in Implantation Failure. In: Franasiak, JM, Scott Jr RT, editors. Recurrent Implantation Failure: Etiologies and Clinical Management. Cham: Springer International Publishing; 2018. p. 21-31.
- Ohara Y, Matsubayashi H, Suzuki Y, Takaya Y, Yamaguchi K, Doshida M, et al. Clinical relevance of a newly developed endometrial receptivity test for patients with recurrent implantation failure in Japan. Reprod Med Biol. 2022;21(1):e12444.
- Mahajan N. Endometrial receptivity array: Clinical application. J Hum Reprod Sci. 2015;8(3):121-9.
- Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1-.e16.
- Elnashar AM. Impact of endometrial microbiome on fertility. Middle East Fertility Society Journal. 2021;26(1):4.
- Busnelli A, Somigliana E, Cirillo F, Baggiani A, Levi-Setti PE. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Sci Rep. 2021;11(1):1747.
- Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419-30.
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169-93.
- ERPeak white paper, CooperSurgical Internal Data, 2021.
- Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74(6):1118-24.
- Taylor M, Pillarisetty LS. Endometritis. Treasure Island (FL): StatPearls Publishing LLC.; 2022.
- Buzzaccarini G, Vitagliano A, Andrisani A, Santarsiero CM, Cicinelli R, Nardelli C, et al. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J Assist Reprod Genet. 2020;37(12):2897-911.

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada