In this webinar, David Chrimes, PhD discussed the physiological and psychological impacts of miscarriage and presented data demonstrating how PGT-A and accurate embryo assessment can help reduce miscarriage rates across all age ranges.
Reducing miscarriage through PGT-A and accurate embryo assessment
In this webinar, Director of Global Genomics Business Development at CooperSurgical, David Chrimes looks at how PGT-A (Preimplantation Genetic Testing for Aneuploidies) can reduce the chances of miscarriage. A molecular biologist by training, David has been working in the genomics testing of embryos since 2008. Prior to that, he worked in the genetic testing of children born with severe learning disabilities and dysmorphia and also in the prenatal testing of abnormal scans.
Because of his background, he approaches this webinar from a human genome genetics testing perspective. The presentation focuses on reducing miscarriage through PGT-A and accurate embryo assessment. It also covers:
- Miscarriage and maternal age
- The psychological impacts of miscarriage
- The physiological impacts of miscarriage
- How miscarriage risk is very much correlated with the risk of aneuploidy
- How the risk of aneuploidy increases with maternal age
- Evidence for how PGT-A utilization for miscarriage reduction is possible across all age ranges of people undergoing IVF
Miscarriage and maternal age
It has been known for many years that aneuploidy is one of the driving causes of miscarriage. A study carried out in 2007 found that up to 70 percent of miscarriage specimens (or “products of conception” as they’re sometimes referred to), showed some form of cytogenetic abnormality.
The vast majority of these were autosomal trisomies. These are your autosomes with the chromosomes shared between both male and female chromosomes 1 to 22. We’ll look at a few of these shortly but they accounted for 60% of trisomies in these spontaneous abortions across the autosome.
Monosomy X was also quite common (20 percent). This is also known as Turner syndrome and while Turner syndrome can result in a live birth, most of them are mosaic. Nonetheless, they’re frequently found in spontaneous abortions.
Finally, polyploidy (where you have either one copy of the genome rather than two, three or more copies) was responsible for a further 20 percent of those found to have some kind of cytogenetic abnormality.
Every couple is at risk of having embryos which are aneuploid. This is, however, correlated to maternal age.
Let’s start by looking at the green data in the graph below, the euploids.
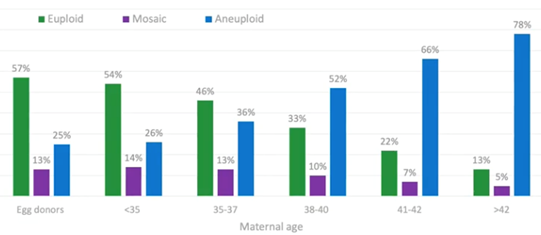
In the under-35s, around 50 percent of embryos available for testing are euploid. These are embryos which have made it to day five or six blastocyst and trophectoderm biopsy. 50 percent of those that are remaining are euploid, and the remaining are either aneuploid or mosaic.
By the time we get to ages 38-40, we’re down to one in three embryos which are truly euploid. When we reach 41-42, we’re down to 22 percent (or, effectively, one in five) embryos being euploid.
We have a corresponding increase in aneuploidy rate with age. You would expect this; if they’re not euploid, there must be an increasing chance of aneuploidy.
There are also the embryos which sit in the middle – the mosaic embryos. These are a mixture of both euploid and aneuploid cells. I discuss this in another webinar, Mosaicism: Everything you Need to Know (1).
What we’re looking at today, however, is the increase in aneuploidy and decrease in euploidy with maternal age and how this impacts miscarriage risk. Again, this has been known for many years and it’s not related to IVF. It’s just a natural phenomenon.
As maternal age increases, the risk of miscarriage increases significantly, especially after the age of 35 onwards.
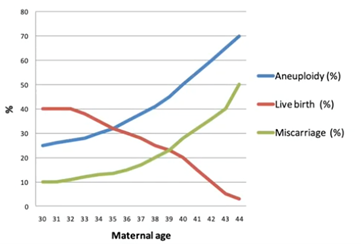
If we look at natural fertility, we know that the chance of having a successful child rapidly declines as maternal age increases. This is being driven both by the increase in miscarriage – but also a decrease in potential of live birth – because of increased aneuploidy in the embryos and eggs.
The most visible and well-documented increase in aneuploidy in human embryos and eggs is trisomy 21, which is Down syndrome. We know that, in the under-30s, your risk of having a Down syndrome-affected child is about one in 800-1,000. By the time you reach 42, this increased to a one-in-four risk.
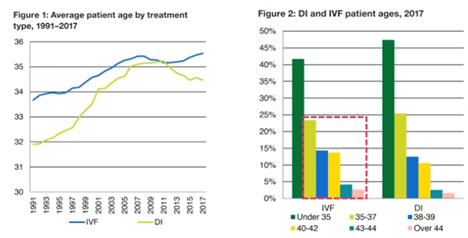
For today’s webinar, I have chosen UK data which is replicated across the world. It shows that both the average maternal age of patients undergoing IVF and the average maternal age of first-time mothers is increasing across most populations.
What we’re seeing here is that from 1991 to 2017, there has been a steady increase in maternal age of patients undergoing IVF, with half now being over 35 (so very much in this increased risk of miscarriage group).
The impact of miscarriage
The impact of miscarriage can be split into two parts – physiological and psychological. We’ll start off by looking at the physiological impact of miscarriage.
The physiological impact of miscarriage
As we’ve discussed, the risk of miscarriage increases with maternal age. It’s estimated that 15-25 percent of recognized pregnancies across all age ranges end in miscarriage.
In some cases, we have what’s termed as a missed miscarriage. This is where the baby has died or failed to develop properly but as yet, it hasn’t been physically miscarried because the body still hasn’t rejected the pregnancy. This tends to be picked up at early scans where the fetal heartbeat is no longer detected.
You can also have an incomplete miscarriage. This is where the body has started a miscarriage but there is still some fetal tissue remaining in the uterus. The cervix is dilated but it’s failing to get rid of all the fetal and placental tissue from the uterus.
In both cases of missed miscarriage and incomplete miscarriage, the patient is faced with a difficult dilemma. There’s the kind of managed approach with either drugs or surgical intervention, or there’s a wait-and-see approach to see if the body can naturally resolve itself.
It’s a difficult choice for patients and one which really puts them under a huge amount of stress.
Whichever way the pregnancy or miscarriage is managed, comes with potential complications.
Even if the miscarriage happens without any intervention whatsoever, you can have excessive bleeding and haemorrhaging. If this happens, the woman loses a lot of blood and sometimes requires a blood transfusion or even invasive surgery.
Around three percent of women will acquire some kind of infection related directly to the miscarriage, the trauma and the blood floating around inside. Unfortunately, the woman’s intrauterine cavity is an ideal breeding ground for bacteria. This can cause an infection which is highly unpleasant.
As I mentioned earlier, in some cases, the patient may require surgery. Dilation and Curettage (D&C) surgery is obviously an invasive procedure. It requires an operation and operations come with risks.
We then have Asherman syndrome which can occur naturally but is quite often seen after D&C surgery. Some reports say Asherman syndrome is present in some form in up to 40 percent of patients who have to undergo D&C surgery.
The psychological impact of miscarriage
Even if you can medically manage the miscarriage, there is psychological trauma for the woman.
A study in 2018 showed that up to 15 percent of women suffer severe depression which can last up to four months post miscarriage. The grief experienced is comparable in nature, intensity and duration to grief reactions in people suffering other types of major loss such as the death of a close relative. This shows how mentally trying it is for women who experience miscarriage.
Another study published at the end of 2019 found that many women also experience Post-Traumatic Stress Disorder (PTSD) following miscarriage. While the PTSD and depression does decline over time, it can still be clinically important even nine months after the miscarriage event.
If you’re feeling traumatized and depressed, you’re not going to want to undergo another embryo transfer, especially with the risks you perceive this may have. The potential management of miscarriages can delay subsequent transfers, or for some women, stop them having another transfer at all.
PGT-A overview: why it’s important for miscarriage reduction
We want to reduce miscarriage and we can do this through PGT-A. So how does PGT-A work? What is the theory behind it and how can it be used?
What is PGT-A?
PGT-A assesses the chromosomal health of an embryo prior to transfer. This means we can check that, to the best of our knowledge, the embryo has the correct number of chromosomes.
By doing this, we can better inform transfer decisions and identify the embryos most likely to lead to a healthy pregnancy. I should be clear, PGT-A is a screening test, it’s not diagnostic and there are other reasons why an embryo may fail to implant.
We’re trying to get all the information we can to make the best embryo transfer decisions possible. By doing this, we improve IVF outcomes, and we help the woman to have a successful transfer and live birth with the least complications.
Chromosomal health of embryos
A euploid embryo is an embryo which has the correct number of chromosomes. This has been shown to have the highest likelihood of successful pregnancy.
In the image below, we have our normal chromosome complement.

Chromosome numbers one to 22 are the autosome chromosomes which are shared between males and females. Then, if you’re female, you have two copies of X and if you’re male, you have an X and a Y.
X and Y are the sex chromosomes and you should have two of them. Overall, your embryo should have 23 pairs of chromosomes.
If you have an aneuploid embryo, it means you don’t have the complete set of 23 pairs. You either have one gained or one (or maybe more) missing.
We know that an aneuploid embryo may result in a failed implantation which means that you don’t get pregnant at all. If you do get pregnant, you have a much higher chance of miscarriage and depending on that genetic abnormality, you may have a genetic disease.
As I showed earlier, aneuploidy is found in embryos of any patient age, but it has been shown to increase with maternal age. What we’ve highlighted below is a trisomy chromosome 10.
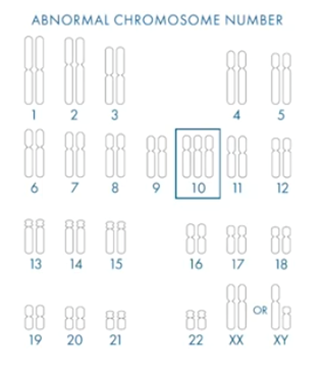
There has never been a reported live birth with trisomy 10. If we find an embryo with a trisomy 10 in it, we know that the chance of it leading to a successful live birth is incredibly low. This is what PGT-A is there to do – to find the embryos with the highest chance of a successful pregnancy.
As many of you are aware, there are also embryos which kind of live in the middle, the mosaic embryos. These are embryos which have two or more cell lines, some of which are euploid and some are aneuploid.
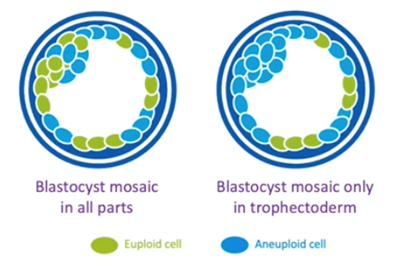
There’s increasing evidence that these mosaic embryos will implant less, and this will depend on the level of mosaicism. They will miscarry more even if they do implant, but they can sometimes lead to a healthy live birth.
PGT-A can accurately stratify the embryos into euploid, aneuploid, and mosaic. This can help make better informed IVF choices and help patients understand the risk associated with that embryo transfer.
Evidence for utilization of PGT-A in miscarriage reduction
If we were to utilize PGT-A and adopt it in our clinic for looking at and screening the embryos, what evidence is there that this utilization will actually reduce the risk of miscarriage?
Below we have an overview of the key randomized control trials. I’ve gone all the way back to the Mastenbroek 2007 (2) because it’s still raised by some people.
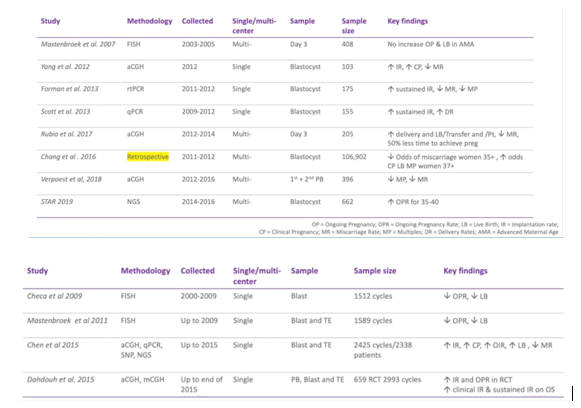
For the latest studies which use newer technology and blastocysts, particularly in multi cell, we’ve seen an increase in implantation rate and a decrease in miscarriage rate.
Almost all of these studies and randomized control trials have shown a decrease in miscarriage rate.
The studies are quite small, so they did a meta-analysis and, again, those using the later technologies such as Array CGH, NGS and SNP technology, have shown an increase in implantation rate.
What we’re seeing is, per embryo transfer, a decrease in miscarriage rate and that’s really where PGT-A is shown to have one of the key benefits.
There are many discussions as to the value of observational data versus randomized control trials. That’s a whole different talk but today I want to go through some of the observational study data that’s out there.
One particular paper (3) looked at advanced maternal age, with the average patient age of 38.
What they found was that implantation rate was very high (80-86 %), live birth rate per embryo transfer was also very high (60-73 %) and clinical miscarriage rate was below 10%.
Remember, this is in patients above 38 years of age on average so to have a miscarriage rate below 10% is stunning. The other thing they highlighted is that this miscarriage was not affected by the embryo morphology, it was the euploid/aneuploid that was driving this score. If you find the euploid, put it back ahead of the morphological scores.
This was another observational study (4) where they tested the embryos and only put back those that were euploid. They did this via single embryo transfer.
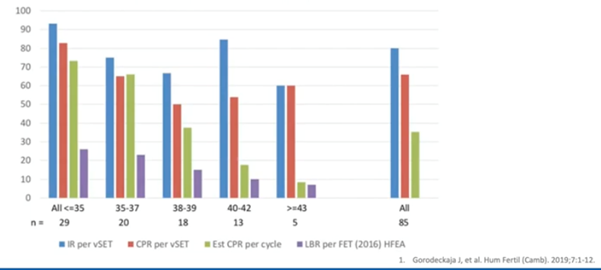
They found that, irrespective of patient age, if you found a euploid embryo, you had a very high implantation rate – much higher than without testing. Again, clinical pregnancy rate was also very high. This meant you had an implantation event and the sustained pregnancy, or a clinical pregnancy rate very much higher than without testing.
This, again, shows a low miscarriage rate if that embryo was shown to be truly euploid prior to transfer.
We also have more observational data (5) from a group in the US who looked over their data from 2014 to 2018. They found many positives to PGT-A but again, for the sake of today’s talk, we’ll look at the miscarriage rate.
In their control group, it was 13 % across all age ranges. This covers every single patient that came into their clinic. 13 percent is still low but, by doing PGT-A, they could reduce it to 4.4 percent. This meant that just 4.4 percent of embryo transfers resulted in miscarriage which is very good news for patients.

Below is a clinical trial (6) published at the end of 2018 which looked at polar bodies only. This meant taking polar bodies from the oocyte to determine if screening out the eggs which are likely to be aneuploid could improve IVF outcomes.
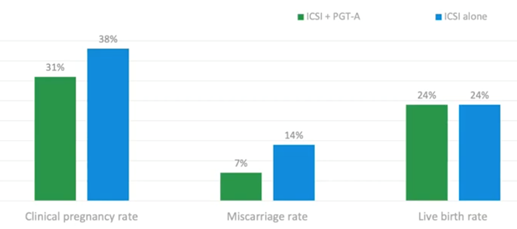
They found that just looking at the maternal (egg) contribution, they could halve their miscarriage rate without affecting the live birth rate per cycle started.
They managed to achieve the same live birth rate per cycle started in patients aged over 35. This was an older patient group, and they could get that with a reduced miscarriage rate and a reduced number of transfers. This meant that women were subjected to fewer transfers and fewer miscarriages, while still maintaining the same live birth rate per cycle started by undergoing PGT-A.
The data below (7) shows an earlier clinical trial on high prognosis patients under 35 years of age.
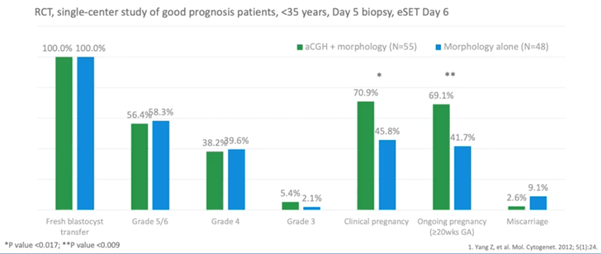
They found a background miscarriage rate of 9.1 percent with the untested embryos, which was very good but, when doing PGT-A, this could be reduced to 2.6 percent.
At the time, this was the largest retrospective cohort study that had been carried out. There were 97,000 non-PGT cycles and nearly 10,000 PGT cycles. For those undergoing PGT-A (specifically aneuploid screening), they found that the odds of miscarriage decreased.
The ‘side effect’, if you will, was increased odds of a live birth (which is great), but also an increase in the likelihood of multiple live birth delivery. This is because, even if they had PGT-A in the study, they weren’t driving for single embryo transfer. There were many transfers which were actually two transfers of euploids.
The key for this is that PGT-A was decreasing the odds of miscarriage.
The largest dataset in the world is from the Society of Assisted Reproductive Technology (SART) in the US. We’ve mined the data from 2014 through to 2016, which includes complete, as well as provisional data up to the end of 2017.
If we look at the miscarriage rate in the untested, you can see that this increase. With PGT-A however, we flattened the curve.
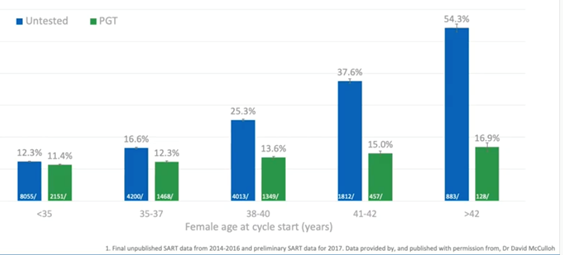
It’s important to note the size of this dataset. There were nearly 450,000 cycles of which nearly 135,000 were PGT-A. We’re getting close to population study here. We have the majority of clinics in the year submitting their data, we’re covering all sorts of patient demographics and all sorts of clinic demographics. A huge number of variables are collated into this data.
What this shows is that the baseline miscarriage risk is around 12.5 percent for high prognosis patients under-35. Even in the under-35s, there was a slight reduction in miscarriage risk with PGT-A. Again however, once we hit the above 35 ages, we really see the increase in miscarriage risk in the untested.
By the time we reach 38 to 40, it goes up to 25 percent. This means that one in four pregnancies will end in miscarriage. If we do this with PGT-A however, it goes down to 13 percent. This is a massive reduction, half the risk basically compared to non-tested.
By the time you reach 41-42, your miscarriage risk is very high in the untested but remains low if you do the testing. This again shows the power of PGT-A to reduce miscarriage risk across whole patient demographics and multiple clinics.
What this is driving is an increased live birth rate per embryo transferred. Ultimately, when we undergo an IVF cycle, we wish for the patient to have a live birth with the fewest transfers possible. This is what PGT-A will give us; it’s basically maintaining around a 50% live birth rate per embryo transfer.
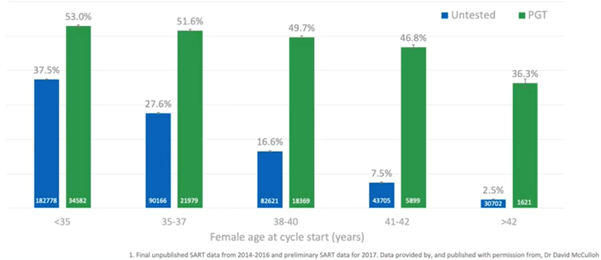
If we don’t do PGT-A and we just transfer the embryos sequentially, by the time we reach the ages of 38 to 40, only one in six transfers will result in a live birth. This means that you’re potentially going through three to four extra transfers compared to doing euploid transfer just to get that live birth.
Even in the 35 to 37 age bracket, you’re going to have to have, statistically, four transfers (double the number of transfers) to get a live birth compared to doing PGT.
By reducing miscarriage and the number of embryo transfers required for a live birth, what we’re now seeing when we look at such large data, is a live birth per retrieval increasing if couples undergo PGT-A.
This is really starting to show significance in the above 35 age range. It’s almost certainly due to the avoidance of unnecessary transfers, not going through the psychological trauma of miscarriage as well as clinics being able to make sure that these patients get the best embryos as early as possible.
There’s also the benefit of reducing the number of preterm deliveries as we can see in the graph below.
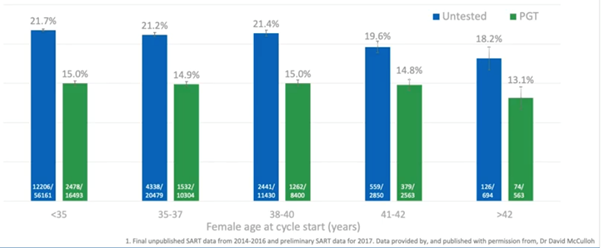
If you have PGT-A, you’re going to be more confident when doing a single embryo transfer. You know that the embryo you’re transferring is euploid with a high chance of success. You’re avoiding the double or triple embryo transfers and we know that twins, (particularly in older patients), have a much higher risk to the mother, and of a preterm delivery.
This is where we start to see another benefit of testing the embryos and making sure we only transfer the highest quality embryos. We’re also reducing the preterm deliveries and the complications associated with them.
Conclusion
We know that miscarriage is highly traumatic for many women and what we haven’t really talked about today and it hasn’t been studied so much, is that it’s also traumatic for men.
Miscarriage is most often caused by chromosomal aneuploidy. This has been shown through many studies which have looked at the product of conception and miscarriage testing.
Increasingly and repeatedly, PGT-A has been shown to mitigate the age-related miscarriage risk. This means the technology is available and ready to help minimize the traumatic experience of miscarriage.
About the Presenter: David Chrimes PhD
David is Director of Global Genomics Business Development at CooperSurgical. Here he helps drive innovation in genomics testing and also gains regular insights to the challenges faced by IVF clinics today.
Find out more about David Chrimes PhD.
Other relevant links:
The following are mentioned throughout the text above and/or are particularly relevant to it. To find out more about each item, please click the link.
Genomics Products:
Procedures
Studies/Webinars Cited:
- CooperSurgical webinar on Mosaicism: Everything you Need to Know
- Mastenbroek et al, In vitro fertilization with preimplantation genetic screening, 2007
- Gonzalez et a, Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients, 2019
- Gorodeckaja et al, High implantation and clinical pregnancy rates with single vitrified-warmed blastocyst transfer and optional aneuploidy testing for all patients, 2019
- Anderson et al, Clinical benefits of preimplantation genetic testing for aneuploidy (PGT-A) for all in vitro fertilization treatment cycles, 2019
- Verpoest et al, Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial, 2018
- Yang et al, Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study, 2012
External Links

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
