SAGE™ Vitrification Solutions
The vitrification kit you need
CooperSurgical’s vitrification products are designed for Embryologists and IVF labs looking for flexible protocols, reliable products, and consistent outcomes.
CooperSurgical’s SAGE Vitrification Kit is a cooling and warming vitrification media kit for all stages that offers maximum flexibility and efficiency to meet most preferences.
Simple, reliable, flexible.
Our SAGE vitrification media kits are a solution for labs looking to simplify vitrification at every stage, supporting embryologists to deliver the best outcomes possible for their patients.
Discover more below or learn about our other Embryo Cryopreservation Products
Vitrification
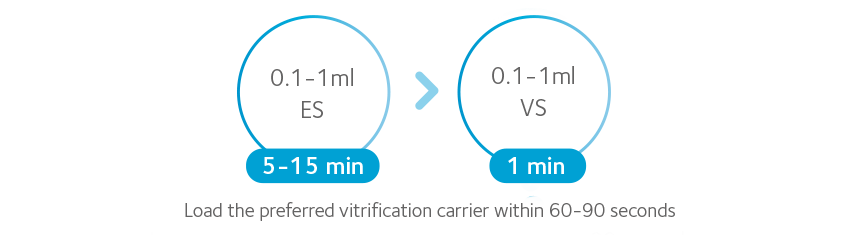
Vitrification kits are designed for up to 40 cases and all steps are performed at room temperature
Vitrification
Vitrification kits are designed for up to 40 cases and all steps are performed at room temperature
Warming
Warming kits available to support up to 32 cases. All steps are performed at 37°C
Availability
This product is available for sale globally.
Key Product Information
Dr Lodovico Parmegiani’s ESHRE Presentation: Universal Warming Protocol for Human Reproductive Cells
Dr Lodovico Parmegiani presents data that demonstrates you can use a universal warming protocol with slow frozen or vitrified embryos or oocytes, that differs from the media and method used for freezing. For example, warming Kitazato-vitrified blastocysts with SAGE media and vice versa.
This optimizes costs, simplifies lab routines and allows embryo exchange between IVF centers. These findings were presented for the first time at the conference.
Webinars
Order Codes
| Reference | Description | Size | HSA mg/ml | Shelf Life |
|---|---|---|---|---|
| ART-8026 | SAGE Vitrification Media Kit includes: Equilibration Solution Vitrification Solution |
2×2 ml 2×2 ml |
12 mg/mL | 1 year from Date of Manufacture |
| ART-8031 | SAGE Vitrification Warming Kit includes: 1.0 M Sucrose Warming Solution 0.5 M Sucrose Warming Solution MOPS Solution |
2×2 ml 2×2 ml 2×2 ml |
12 mg/mL | 1 year from Date of Manufacture |
| ART-8034 (US ONLY) | SAGE Vitrification Warming Kit includes: 1.0 M Sucrose Warming Solution 0.5 M Sucrose Warming Solution MOPS Solution |
8×2 ml 2×2 ml 2×2 ml |
12 mg/mL | 1 year from Date of Manufacture |

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada