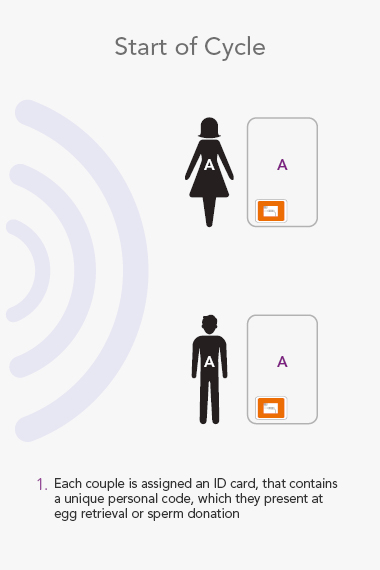
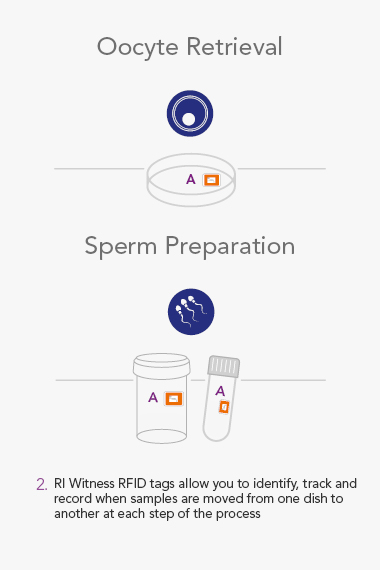
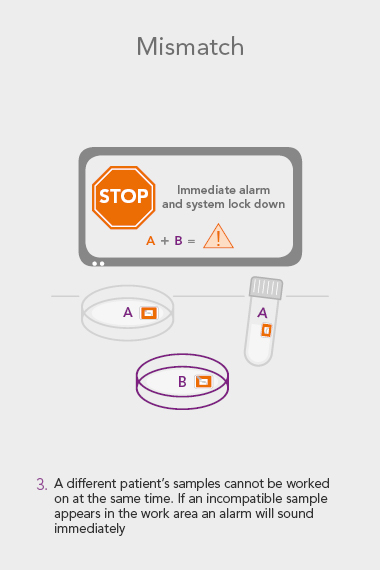
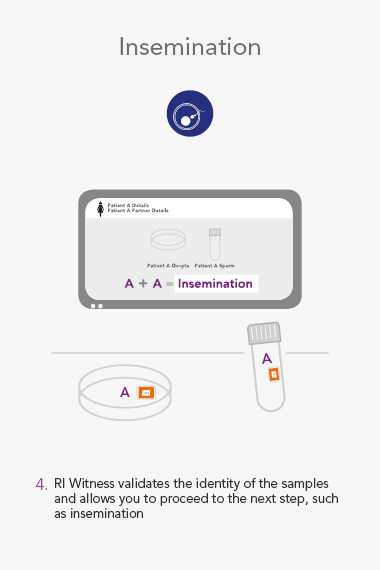
RI WitnessTM ART Management System
Electronic witnessing is now an established and proven methodology that has led it to become the global gold standard for mismatch avoidance.

Since 2007, at the forefront of the industry movement towards increased visibility and accountability, we designed, alongside embryologists, a system that gives you increased confidence in your everyday practises. RI Witness is now the world’s most established and trusted ART electronic witnessing system, installed in over 40 countries across six continents.
In addition to the core undertaking of mismatch avoidance, RI Witness offers benefits in quality control, traceability, workflow efficiency and reduced administration. Having RI Witness in your clinic builds on the trust your patients have in you. It helps you and your team have confidence that everything is as secure and efficient as possible
What is RI Witness?
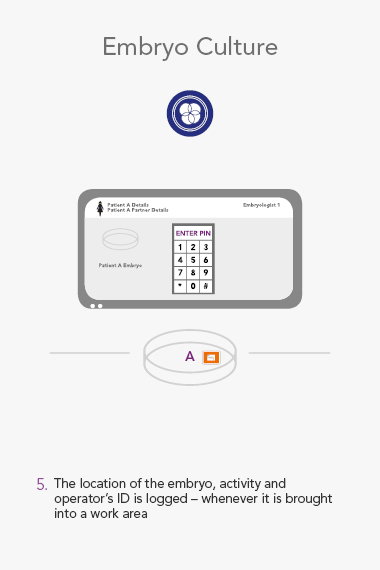
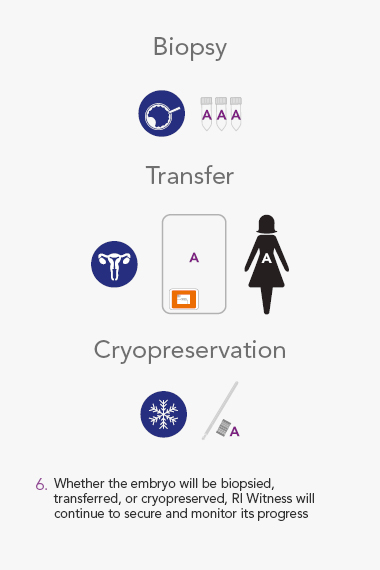
RI Witness uses Radio Frequency Identification(RFID) to detect and monitor all activity in the IVF Laboratory. The system helps mitigate the risk of human error every time samples are moved from one dish or tube to another, and safeguards every step of the IVF cycle.
Throughout the lab, RI Witness readers are situated wherever work is undertaken, critically where samples are handled. Embryology heated or unheated plates with in-built RFID readers can be integrated into a worktop. They are active all day, every day, so a check cannot be overlooked.
 An RI Witness work area has a tablet computer connected to the server, integrated with your clinic’s patient database*. Thanks to self-adhesive RFID tags attached to all the laboratory plasticware, it is possible to ensure protocols are followed and only compatible samples are worked on at any one time. The system is automatic, so it does not require additional steps by embryologists to identify samples, which are necessary with human double witnessing or barcode witnessing systems. By automatically tracking, monitoring and recording across all your work areas, RI Witness frees up your time, energy and expertise. This secured process fills everyone with total confidence2.
An RI Witness work area has a tablet computer connected to the server, integrated with your clinic’s patient database*. Thanks to self-adhesive RFID tags attached to all the laboratory plasticware, it is possible to ensure protocols are followed and only compatible samples are worked on at any one time. The system is automatic, so it does not require additional steps by embryologists to identify samples, which are necessary with human double witnessing or barcode witnessing systems. By automatically tracking, monitoring and recording across all your work areas, RI Witness frees up your time, energy and expertise. This secured process fills everyone with total confidence2.
*Subject to compatibility, some programming may be required
Why Do I Need RI Witness? – What if you had a mix-up?
Many embryologists tell us this concerns them, and from time to time, the media reports an error which has wide-reaching effects; not only on the family but also the embryologists, the lab and the wider community’s perception of IVF practices. Primarily, RI Witness was designed to mitigate this risk. Through constant monitoring it reduces the chance of human error associated with repetitive tasks and misperception1,3. Compared to other witnessing protocols it involves less disruption and accelerates the speed of witnessing which can reduce the time an embryo is out of the incubator3.
In response to ongoing customer feedback, RI Witness has been developed to include many time-saving features to make laboratory work easier. RI Witness does the work of many people, potentially freeing up staff members to carry out more procedures, increasing overall laboratory efficiency. It can also be used as a quality management tool ensuring consistent adoption of laboratory protocols and monitoring staff performance and simplifying audits. Using RI Witness, provides your clinic with a competitive advantage while offering a secure, patient-orientated service.
What RI Witness is designed to do
Electronic Witnessing was primarily designed for risk reduction
Continual automatic witnessing
- Locks patient identity to every gamete, oocyte, embryo, biopsy and cryo sample for continuous monitoring
- Accelerates ID witnessing throughout the patient cycle
- Active all day, every day: No process goes unchecked

How Does It Work?
Not just for security
RI Witness offers additional values including workflow management for efficiency, time saving and simpler audits
Reduce administration
- Supports audit activity and changes to documentation
- Records and organizes consumables cross-referencing patient cycles and material batches for traceability
Time saving per procedure without a double witness
- Potentially reduces each embryo’s time outside the incubator by decreasing the time spent waiting for a manual witness4
- Supports and guides staff through clinic SOPs, and accelerates staff training
Keep everyone informed
- Easily accessible data displayed in the lab show every cycle’s progress at a glance
- Uninterrupted workflow and communication for the team
Manage and analyze your workflow
- Oversees lab activity in real time
- Allows comparison of lab efficiencies and performance data from multiple labs
- Assigns accountability
- Identifies your training requirements
- Standardizes your procedures across workstations or multiple labs
- Reports workflow bottlenecks so you can increase efficiency
Collects patient cycle information
- Paperless data capture via a tablet touchscreen saves time4
- Direct data input minimises transcription errors by connecting to your patient database
Product Specifications
| Work Areas | One work area required for each critical working location. Microsoft Windows based PC or Tablet needed for each work area. Readers available heated or unheated. RFID reader frequency: 13.56MHz |
| Barcode Compatibility (Traceability) | Compatible with GS1 barcodes (GS1-128) |
| Barcode Scanner (Traceability) | Compatible with USB (Keyboard wedge) fixed and hand held scanners |
| Camera Compatibility (Imaging) | Research Instruments’ DC1 & DC2, Analogue cameras |
| RI Witness™ Manager (Client Software) PC System Requirements | Operating Systems: Windows 11, Windows 10 |
| Server / Network Requirements | Microsoft SQL Server required (not supplied). Network Point required for each work area |
Order Codes
The Order Codes for RI Witness™ will depend on your particular configuration.
Brochures
RI Witness IQ Solution Brochure -German
RI Witness IQ Solution Brochure – French
RI Witness IQ Solution Brochure – Italian
RI Witness IQ Solution Brochure – Spanish
RI Witness IQ Solution Brochure – Turkish
RI Witness Patient Flyer – US
RI Witness Patient Flyer – Turkish
RI Witness Patient Flyer – Portuguese
RI Witness Patient Flyer – Czech
RI Witness Patient Flyer – Greek
RI Witness Patient Flyer – Spanish
RI Witness Patient Flyer – Dutch
RI Witness Patient Flyer – German
RI Witness Patient Flyer – French
RI Witness Patient Flyer – Italian
RI Witness Patient Flyer – Arabic
RI Witness Patient Flyer – Bulgarian
RI Witness Patient Flyer – Danish
Same Sex Couple RI Witness Patient Flyer
RI Witness Patient Flyer – Same Sex Couple Male
RI Witness Patient Flyer – Same Sex Couple Female
RI Witness Patient Flyer – Same Sex Couple Female – Spanish
RI Witness Patient Flyer – Same Sex Couple Female – Czech
RI Witness Patient Flyer – Same Sex Couple Male – Czech
RI Witness Patient Flyer – Same Sex Couple Female – French
RI Witness Patient Flyer – Same Sex Couple Female – Portuguese
RI Witness Patient Flyer – Same Sex Couple Male – Portuguese
RI Witness Patient Flyer Same Sex Couple Male – Bulgarian
RI Witness Patient Flyer Same Sex Couple Female – Bulgarian

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada