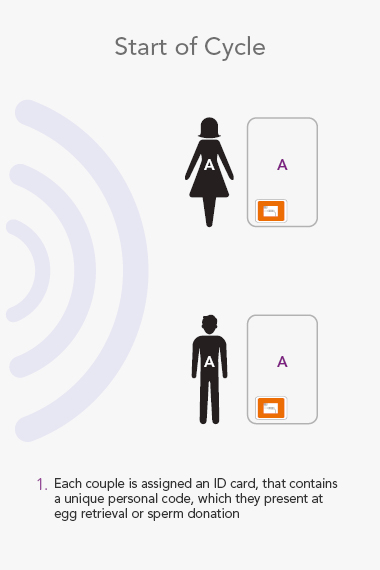
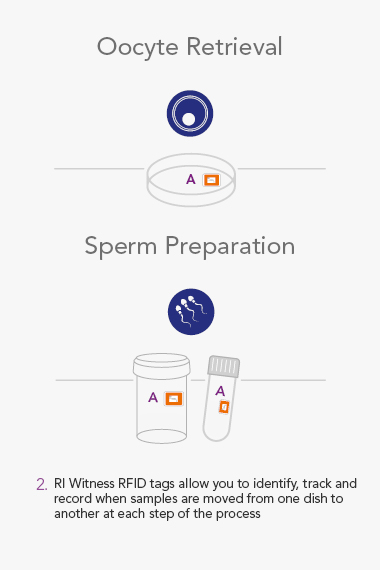
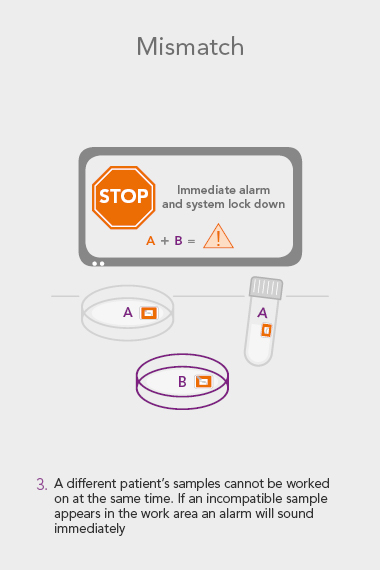
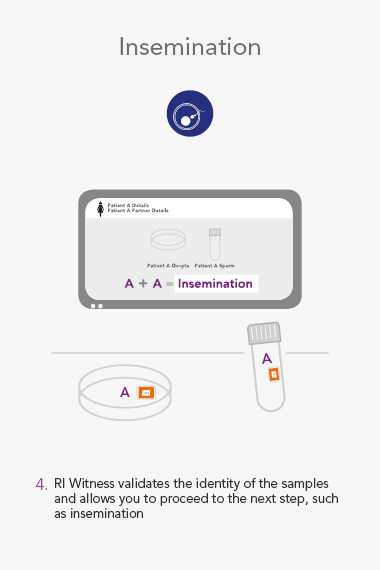
ART Management System
Since 2007, RI Witness has been helping to prevent potential errors and managing activity in IVF laboratories all over the world.
Using RI Witness means that laboratory staff members, fertility clinics and IVF patients enjoy extra peace of mind as well as additional benefits in efficiency and potential cost savings.
For larger clinics and clinic groups, RI Witness technology can be used as a versatile quality control tool to oversee a large team, high volumes of work, data collection and auditing1.
Equally, smaller clinics can use it to help establish accountability and reduce liability; for the first time clinics have the ability to evidence what happened in a patient cycle, even if an embryologist is working alone.
RI Witness works as a constant safeguard to help minimize stress and interruptions, protecting and managing every aspect of your daily workload.

“Since I started using RI Witness in my laboratory the embryologists sleep better and so do the patients.”
Jean–Claude Jacquet, Medical Biologist in Reproduction,
Oriade PMA Grenoble, Rhône Alpes, France
What is RI Witness?
RI Witness uses Radio Frequency Identification(RFID) to detect and monitor all activity in the IVF Laboratory. The system helps mitigate the risk of human error every time samples are moved from one dish or tube to another, and safeguards every stop of the IVF cycle.

Throughout the lab, RI Witness readers are situated wherever work is undertaken, critically where samples are handled. Embryology heated or unheated plates with in-built RFID readers can be integrated into a worktop.
They are active all day, every day, so a check cannot be overlooked.
An RI Witness work area has a tablet computer connected to the server, integrated with your clinic’s patient database*. Thanks to self-adhesive RFID tags attached to all the laboratory plasticware, it is possible to ensure protocols are followed and only compatible samples are worked on at any one time.
The system is automatic, so it does not require additional steps by embryologists to identify samples, which are necessary with human double witnessing or barcode witnessing systems.
By automatically tracking, monitoring and recording across all your work areas, RI Witness frees up your time, energy and expertise.
This secured process fills everyone with total confidence2.
*Subject to compatibility, some programming may be required

Why Do I Need RI Witness?
What if you had a mix-up?
Many embryologists tell us this concerns them, and from time to time, the media reports an error which has wide-reaching effects; not only on the family but also the embryologists, the lab and the wider community’s perception of IVF practices. Primarily, RI Witness was designed to mitigate this risk. Through constant monitoring it reduces the chance of human error associated with repetitive tasks and misperception1,3.
Compared to other witnessing protocols it involves less disruption and accelerates the speed of witnessing which can reduce the time an embryo is out of the incubator3.
In response to ongoing customer feedback, RI Witness has been developed to include many time-saving features to make laboratory work easier. RI Witness does the work of many people, potentially freeing up staff members to carry out more procedures, increasing overall laboratory efficiency. It can also be used as a quality management tool ensuring consistent adoption of laboratory protocols and monitoring staff performance and simplifying audits.
Using RI Witness, provides your clinic with a competitive advantage while offering a secure, patient-orientated service.
«RI Witness has improved efficiency by streamlining our working practice — laboratory procedures are performed at the optimum time with a real-time witness.
The ease of operation is great and the system has proved to be a versatile audit tool.»
Sophie Jewitt, Senior Clinical Embryologist
Gateshead Fertility Unit, England
How Does It Work?
RFID versus Barcodes
RI Witness uses RFID technology instead of barcodes to constantly safeguard the IVF process, from start to finish. Only RI Witness can monitor every procedure with no workflow interruptions.
Multiple Dishes
RFID
RI Witness reads all dishes simultaneously and automatically allowing the operator to work uninterrupted.
Barcodes
Barcodes need to read each each dish, lined up or individually in the line of sight for the scanner to verify that they are compatible.
Samples will need to be taken out of the work area to be checked, which can be disruptive.
Recording Activities
RFID
RI Witness monitors the work area under the microscope constantly. Giving 24/7 protection, a check cannot be unintentionally overlooked.
Barcodes
Barcodes security relies on the operator remembering to do checks. In fact, a procedure can be performed without the activity being logged int he system, no alert or action will be instigated. The IVF cycle’s security could be compromised.
Mismatches
RFID
RI Witness will alert the operator immediately, before samples are manipulated, if incompatible samples are in the same work area. This allows corrective action to be taken while the security of the sample is intact.
Barcodes
Using a human or barcode witnessing system, two unrelated samples can be in the same work area, and may even be manipulated, without the operator being aware that the samples are incompatible.
Perceptual blindness, tiredness, involuntary automaticity5, ambiguous accountability, conscious automaticity and interrupted workflow can all lead to potential mistakes.
Safety of RFID and RI Witness
We understand it is imperative the conditions in your laboratory are optimized for ART procedures. There is no room for compromise. That is why we use technology that does not harm embryos or humans.
Tested
We asked an independant test centre, the University of Liège, Belgium, to devise a comprehensive experiment to prove out theories about the safe use of RFID with gametes and embryos.
For the test they:
- Increased the output power of the reader by 10x
- Increased the time that the embryos were in the radio frequency field by 70 times what would be expected in an IVF cycle
- In the experiment total exposure was 700x what would be expected in an IVF lab
Results:
- Normal development to mouse blastocyst compared to the control group
- Normal live birth rate (LBR) compared to the control group
- Normal fertility in the female pups with normal 2nd generation LBR
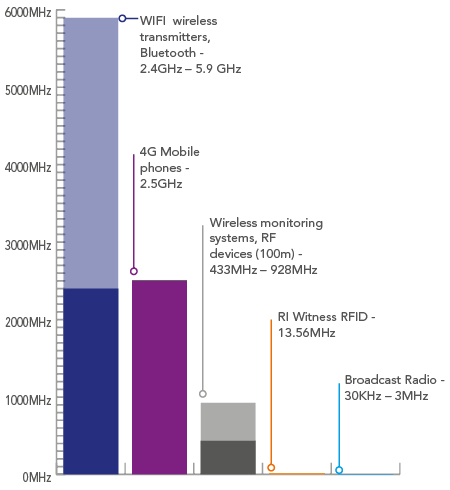
RFID
Every day, we are surrounded by radio frequency emissions from modern technology.
The potential effects radio frequencies have on cells and tissues depend on the frequency and power density to which they are exposed.
The facts:
- RI Witness RFID tags do not emit or receive radio waves when they are not on a reader or in the incubator
- The tags are passive, with no internal energy source, so they are only activated when in a 1-5cm range of an RI Witness reader
- Embryos and gametes are only exposed to the radio frequency fields for short time periods

Statistical Evidence
A four-year statistical analysis in one clinic looked at more than 10,000 live birth, before and after the introduction of RI Witness. The analysis showed no changes to results in both live births and malformations over the two periods7.
Certified
Our embryology heated plates are CE marked as medical devices (GB98/13044) and FDA cleared. They offer highly stable heating across the reader.
Our tags and labels are batch tested to MEA >80% survival to blastocyst stage6.
“Numerous patients have informed us that they feel better knowing that this safeguard, RI Witness, is in place.”
Laboratory Director, Overlake Reproductive Health, USA

A Proven and Validated System
Thanks to widespread clinical use of our Electronic Witnessing System (EWS) for over 10 years, multiple posters and papers have been published that cover and discuss questions often raised about RI Witness.
The Real Risk of Human Error
What is the actual risk of mismatches, for labs relying on human double witnessing, without an electronic system to ensure the cycle is secure?
Using RI Witness, several laboratories analysed data from witnessed steps to establish a “true rate of human error”. The studies found their mismatch rate was up to 0.11%1 of witnessed actions. A true mismatch involves mistaking an identity check, other errors, such as administration errors, were discounted.
This is the first time true errors rates have been studied in an IVF laboratory1.
Efficiency In The Lab
How can RI Witness make my lab more efficient?
Human double witnessing means a colleague must double check the identity of patient samples at critical points. This has been mandatory in the UK since 2004.
Since then, extensive comparisons have been undertaken to compare human, barcode and the RI Witness RFID systems. The evidence concluded that RI Witness was faster, more efficient, and ensured samples were out of the incubator for less time overall4 when compared to human double witnessing.
Staff and Patients’ Considerations
Assessing how the implementation of an Electronic Witnessing System might affect your patients and embryologists is very important. Evidence shows they feel the benefits of using the system in a short space of time.
Patient Reassurance
How might my patients feel about the use of an electronic witnessing system?
One study found that patients and embryology teams felt reassured when using RI Witness, and that it could be used as a trusted strategy to decrease patient stress concerning mismatches; 97.1% of patients confirmed they were more reassured if an Electronic Witnessing System was used in their clinic8.
Key Staff Concerns
Will the installation of an Electronic Witnessing System be disruptive?
The installation of RI Witness and the staff training required takes less than a week to complete, under normal circumstances. A testing and validation phase of one month follows, after installation. A study conducted in an Italian clinic found system users were 96% satisfied with its performance immediately after validation3.
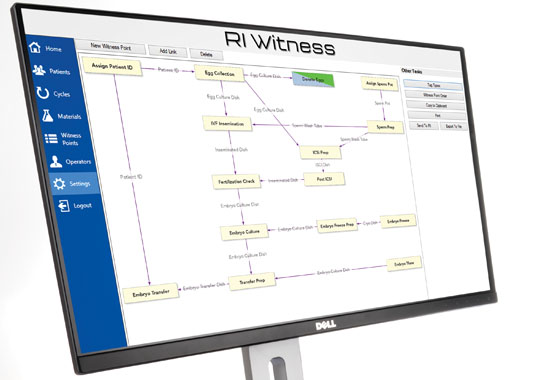
Bespoke Protocol Management
As part of the installations, a bespoke flow chart (Witness Point Diagram) is created with the assistance of a CooperSurgical installation specialist.
It is designed by you and works in line with your existing Standard Operating Procedures (SOPs). Each critical step carried out by every operator, wherever they are in the laboratory, is logged in a manner compatible with your working practices.
The Witness Point Diagram is then tested step by step.
The easy-to-use software interface allow you to amend the diagram as your SOPs and needs evolve.
You are in control, so there are no delays such as waiting for outside assistance.
Whole Lab Integration
Every process in every part of the lab is automatically detected, logged and protected.
Before installation, together we create a plan that outlines your exact requirements including the mapping out of locations for readers and server connections.
Integration
We can fit embryology heated plates into all workstations from major suppliers; flush fitted integration is possible with K-Systems and ORIGIO cabinets.
Work areas can be connected through Wi-Fi or LAN cables to a central server which means everything works together and data updates in real time.
Our software works with leading patient management databases.
We also work closely with clinics and their database administrators on an individual basis to ensure smooth integration with RI Witness.
“The integration of RI Witness into our daily routine was fast and simple and allowed for an improvement in system usage after a short period of time”
Dr Roberts Maggiulli, Biologist Laboratory Coordinator, Clinica Genera, Italy
Additional Functionality
Use RI Witness to its full potential*
On top of the security advantages with RI Witness, the bespoke infrastructure can also be used to improve other aspects of your laboratory’s daily work load.
*Some of these features require additional purchases
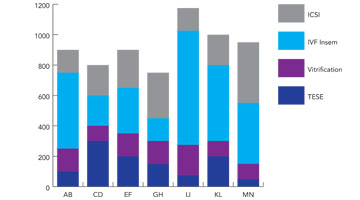
Workflow Management Tool
You can look at the activity being done in the lab, how long it takes, where it takes place and who performed it. This information may indicate that staff training is needed, that there are bottle necks in the process or that equipment requires upgrading or replacing. All of this data allows you to make informed decisions about laboratory work improvements.
Procedure data can be explored in detail or summarized into pie or bar charts.
Cryopreservation Tracking
Patient samples can be tracked as they enter and leave cryo storage.
The RI Witness RFID system converts data to cryo-suitable barcode identification labels. Durable, laboratory-proven Brady labels are used to identify the vials or vitrification straws.
On thawing/warming, the barcode information is linked automatically with the embryo thaw dish.
Paperless Data Capture
You can enter data directly into patient records using small touch screen displays or a keyboard and mouse interface installed at your work areas.
By inputting directly, transcription errors are minimized and multiple data handling is avoided.
Data can be accessed and analyzed immediately.
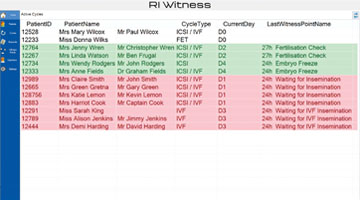
Daily Overview Board
Using a Cycles Overview Display you can view active cycles in the lab. or each cycle the last procedure and current status for the day is shown, for example, in progress, finished or not started.
This information is displayed on a big screen fixed to the lab wall and can be accessed from any networked PC.
The entire team gains an overview of the lab’s daily activities and how the day’s work is progressing.

Materials Traceability
You can record which products are used for each cycle type.
Records can be sorted and filtered in various ways to cross-reference patient cycles and material batches.
It allows you to keep a full record of consumables used in the laboratory, which is often a requirement of regulatory authorities.

Imaging Function
Capture images and videos from every microscope in the laboratory, at any stage of the patient cycle. Information and images for each patient’s cycle can be saved in the patient’s file and accessed immediately from any networked PC.
Patient Display Screen
Show the patient their embryo pre-transfer while checking it against their patient ID card in the transfer room. This enables the patient to be directly involved.